I'm not a class action lawyer. Not a guy with TV ads across networks. Our firm works for people one person at a time to get justice. Here are the posts ... so far:
Oct. 2012: Steroid Injections to blame for Meningitis outbreak?
Making the news in the South are reports of a meningitis outbreak claimed to be related to steroid injections. Our office is investigating incidents, 404-451-7781 or toll free 855-525-3955.
The steroid medication suspected in an outbreak of a rare kind of meningitis was shipped to 23 states, according to the Centers for Disease Control. So far there are reports that a pharmacy that made the steroid, New England Compounding Center of Framingham. There were three recalled three lots of the drug last week and the company has closed.
Facilities that have received and pulled from use the potentially contaminated steroid product include :
The steroid medication suspected in an outbreak of a rare kind of meningitis was shipped to 23 states, according to the Centers for Disease Control. So far there are reports that a pharmacy that made the steroid, New England Compounding Center of Framingham. There were three recalled three lots of the drug last week and the company has closed.
Facilities that have received and pulled from use the potentially contaminated steroid product include :
Berlin Interventional Pain Management, Berlin, MD
Box Hill Surgery Center, Abingdon, MD
Greenspring Surgery Center, Baltimore, MD
Harford County Ambulatory Surgery Center, Edgewood, MD
Maryland Pain Specialists, Towson, MD
SurgCenter of Bel Air, Bel Air, MD Zion Ambulatory Center, Baltimore, MD
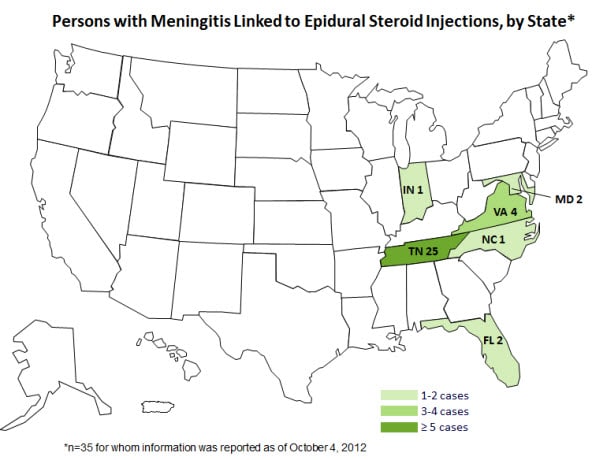
Here is an image of cases by state (Source: http://www.enewspf.com/latest-news/health-and-fitness/37178-cdc-reports-multi-state-meningitis-outbreak-oct-4-2012.html)

What is meningitis:
Meningitis is an inflammation of the membranes (meninges) surrounding your brain and spinal cord, usually due to the spread of an infection. The swelling associated with meningitis often triggers the "hallmark" signs and symptoms of this condition, including headache, fever and a stiff neck in anyone over the age of 2.
Here's a letter from 2006 involving this same company and its compounding practices:
On September 23, 2004, investigators from the U.S. Food and Drug Administration (FDA) and the Massachusetts Board of Pharmacy inspected your firm, located at 697 Waverly Street, Framingham, Massachusetts. On January 19, 2005, the inspection was completed. This inspection revealed that your firm compounds human prescription drugs in various dosage forms and strengths.
We acknowledge the receipt of your October 1, 2004, letter addressed to FDA's New England District Office, concerning questions presented during the referenced inspection.FDA's position is that the Federal Food, Drug, and Cosmetic Act (FDCA) establishes agency jurisdiction over "new drugs," including compounded drugs.
FDA's view that compounded drugs are "new drugs" within the meaning of 21 U.S.C. § 321(p), because they are not "generally recognized, among experts . . . as safe and effective," is supported by substantial judicial authority. "The FDCA contains provisions with explicit exemptions from the new drug . . . provisions. Neither pharmacies nor compounded drugs are expressly exempted."). FDA maintains that, because they are "new drugs" under the FDCA, compounded drugs may not be introduced into interstate commerce without FDA approval.
The drugs that pharmacists compound are not FDA-approved and lack an FDA finding of safety and efficacy.However, FDA has long recognized the important public health function served by traditional pharmacy compounding. FDA regards traditional compounding as the extemporaneous combining, mixing, or altering of ingredients by a pharmacist in response to a physician's prescription to create a medication tailored to the specialized needs of an individual patient . See Thompson v. Western States Medical Center, 535 U.S. 357, 360-61 (2002).
Traditional compounding typically is used to prepare medications that are not available commercially, such as a drug for a patient who is allergic to an ingredient in a mass-produced product, or diluted dosages for children.
Through the exercise of enforcement discretion, FDA historically has not taken enforcement actions against pharmacies engaged in traditional pharmacy compounding. Rather, FDA has directed its enforcement resources against establishments whose activities raise the kinds of concerns normally associated with a drug manufacturer and whose compounding practices result in significant violations of the new drug, adulteration, or misbranding provisions of the FDCA.FDA's current enforcement policy with respect to pharmacy compounding is articulated in Compliance Policy Guide (CPG), section 460.200 ["Pharmacy Compounding"], issued by FDA on May 29, 2002 (see Notice of Availability, 67 Fed. Reg. 39,409 (June 7, 2002)).1 The CPG identifies factors that the Agency considers in deciding whether to initiate enforcement action with respect to compounding.
These factors help differentiate the traditional practice of pharmacy compounding from the manufacture of unapproved new drugs. They further address compounding practices that result in significant violations of the new drug, adulteration, or misbranding provisions of the FDCA. These factors include considering whether a firm compounds drugs that are copies or essentially copies of commercially available FDA-approved drug products without an FDA sanctioned investigational new drug application (IND). The factors in the CPG are not intended to be exhaustive and other factors may also be appropriate for consideration.
10/16/12 Steroid Injection Recall News: New Drugs (Cardioplegic Solution) Tied to Meningitis Outbreak
From the FDA, more worrisome news:
ISSUE: As a result of the ongoing investigation of New England Compounding Center (NECC), a patient with possible meningitis potentially associated with epidural injection of an additional NECC product, triamcinolone acetonide, has been identified through active surveillance and reported to FDA. Triamcinolone acetonide is a type of steroid injectable product made by NECC. The cases of meningitis identified to date have been associated with methylprednisolone acetate, another similar steroid injectable product.
In addition, two transplant patients with Aspergillus fumigatus infection who were administered NECC cardioplegic solution during surgery have been reported. Investigation of these patients is ongoing; and there may be other explanations for their Aspergillus infection. Cardioplegic solution is used to induce cardiac muscle paralysis during open heart surgery to prevent injury to the heart.
From the FDA, more worrisome news:
ISSUE: As a result of the ongoing investigation of New England Compounding Center (NECC), a patient with possible meningitis potentially associated with epidural injection of an additional NECC product, triamcinolone acetonide, has been identified through active surveillance and reported to FDA. Triamcinolone acetonide is a type of steroid injectable product made by NECC. The cases of meningitis identified to date have been associated with methylprednisolone acetate, another similar steroid injectable product.
In addition, two transplant patients with Aspergillus fumigatus infection who were administered NECC cardioplegic solution during surgery have been reported. Investigation of these patients is ongoing; and there may be other explanations for their Aspergillus infection. Cardioplegic solution is used to induce cardiac muscle paralysis during open heart surgery to prevent injury to the heart.
The Tennessee Department of Health has now confirmed that 74 healthcare facilities in Tennessee have received suspect material from New England Compounding Center that may pose a risk to some patients. The Food and Drug Administration has characterized the notification effort as recommended “out of an abundance of caution.”
How those facilities were identified:
The evening of Monday, Oct. 15, the FDA provided TDH a list of more than 131,000 shipping invoices for NECC materials that went to medical facilities across the U.S. TDH staff members began work immediately to identify which NECC invoices were for materials going to Tennessee. On Oct. 16, TDH staff members were able to confirm where the majority of shipments had gone and today continue combing the invoices for additional data.
The evening of Monday, Oct. 15, the FDA provided TDH a list of more than 131,000 shipping invoices for NECC materials that went to medical facilities across the U.S. TDH staff members began work immediately to identify which NECC invoices were for materials going to Tennessee. On Oct. 16, TDH staff members were able to confirm where the majority of shipments had gone and today continue combing the invoices for additional data.
The process to notify patients:
Also on the evening of Oct. 15, TDH asked for and received cooperative assistance from the Tennessee Hospital Association, the Tennessee Medical Association, the Tennessee Pharmacy Association, AMSURG and other healthcare organizations. Through a cooperative effort, TDH and its partners began contacting healthcare facilities today, providing information to help each facility with an action plan for contacting their patients. The facilities are being urged to identify which patients received potentially unsafe NECC products during heart or eye surgeries and to then contact their patients to provide information about the risk for infection. TDH anticipates some patients will begin receiving contacts as early as this week.
Also on the evening of Oct. 15, TDH asked for and received cooperative assistance from the Tennessee Hospital Association, the Tennessee Medical Association, the Tennessee Pharmacy Association, AMSURG and other healthcare organizations. Through a cooperative effort, TDH and its partners began contacting healthcare facilities today, providing information to help each facility with an action plan for contacting their patients. The facilities are being urged to identify which patients received potentially unsafe NECC products during heart or eye surgeries and to then contact their patients to provide information about the risk for infection. TDH anticipates some patients will begin receiving contacts as early as this week.
TDH and its partners are working with healthcare providers on the important patient identification and contact efforts. To allow time for these facilities to put their notification plans in place, TDH and its partners are providing assistance with necessary processes at all facilities. TDH estimates this work will occur very rapidly. The department respects and appreciates care providers’ desires to be the first to contact their patients about the potential risk as an important part of their clinician-patient relationship.
10/17 Florida/USA - Steroid Recalled Medications - Facilities List
Here is the list of of Florida facilities, and a list of facilities across the country below
- Florida Pain Clinic, Ocala, FL
- Marion Pain Management Center, Ocala, FL
- Orlando Center for Outpatient Surgery, Orlando, FL
- Pain Consultants of West Florida, Pensacola, FL
- Surgery Center of Ocala, Ocala, FL
- Surgical Park Center, Miami, FL
10/19: Alabama Meningitis Outbreak News- NECC Steroid Medications
Alabama health officials say two Alabamians are showing symptoms of meningitis after receiving tainted steroid injections. Officials have contacted four of the six additional people who also received the shot.
All six are Alabama residents who were treated in Florida, and thirteen other Alabamians were treated in Florida and Tennessee. The Alabama residents who have contracted meningitis from the contaminated drug have received the medication from outside of the state.
Now, authorities are looking at other medicines from the New England Compounding Center to see if they are safe. Health officials in both Alabama and Florida started contacting health care facilities in their states who have received such medications, but only medicines that were sent to them since January.
The clinics and physicians are being urged to then notify their patients who might have received them, especially any patients who might have been treated with injections for eye or heart surgeries.
10/26/12: Tainted Steroid News: NECC's Inspection Report from 2011
NECC, which made the steroid suspected in a deadly meningitis outbreak was inspected and cleared by Massachusetts inspectors last year. The inspection by the Department of Public Health was required after the New England Compounding Center in Framingham relocated its pharmacy on the same site.
Here is the report
03 new-england-compounding-pharmacy-incnew-england-coumpouding-center-inspection-report from mzamoralaw
10/30: Texas Facilities Received Tainted Steroid Meds
Texas Health Harris Methodist Hospital in Southlake has previously acknowledged receiving tainted medicine from the New England facility but says it immediately pulled the drugs and notified patients.
Other North Texas hospitals that are included on the FDA's new list as receiving other medicine from the compounding center include:
- Childrens Medical Center in Dallas. A spokesman says it treated 34 patients with amino acids not linked to any illness, and notified all of them as a precaution.
- Cook Childrens Medical Center in Fort Worth. The hospital said Wednesday that a cleanser, Glutaraldehyde, that is used to disinfect medical equipment is the only product it received from the New England Compounding Center. The hospital said it did not contact patients about the cleanser but sent the chemical back to the New England Compounding Center.
- Medical City in Dallas. The hospital immediately pulled all drugs it bought from the New England Compounding Center and is following up with patients, spokeswoman Chris Hawes said.
- Medical Center of Arlington. The hospital said it immediately pulled the medicine in question as soon as it learned of the recall but a "small number" of patients had already received doses. "While these medications have not currently been confirmed as causing infections and authorities believe the risk is very low, we are in the process of notifying these patients out of an abundance of caution," the hospital said in a statement.
- UT Southwestern Medical Center in Dallas. "UTSW had a small inventory of the topical cream, which was pulled as soon as the FDA advisory came out," the hospital said in a statement.
- Huguley Memorial Medical Center in Burleson. Pharmacy Director James Hall said it got 100 doses of a drug used to help digestion, which were all used on patients. Hall said there is no reason to believe anything was wrong with the doses but is contacting patients. "At this point, we're communicating in writing to any patient who might have received the product we bought from the NECC. ... We've quarantined a supply from a partner company Ameridose," he said. "We're waiting on steps to return it or dispose of it."
- Plaza Medical Center in Fort Worth. The hospital said none of the drugs it got from the New England Compounding Center are among those said to be contaminated. The hospital said it sent letters to patients to let them know where the drug they took came from and to tell them they are fine. Plaza Medical Center said the drugs are locked up and will not be used.
http://www.nbcdfw.com/news/health/Voluntary-Drug-Recall-Widespread-in-North-Texas-175527951.html
10/31 FDA: Ameridose Recalls All Products
From Reuters:
Ameridose, a sister company of the pharmacy linked to a meningitis outbreak that has killed 28 people, announced Wednesday a voluntary recall of all its products, a move to cooperate with regulators that could nevertheless create shortages of some drugs.
In particular, the Food and Drug Administration is concerned about the availability of several drugs given as shots or intravenous drips or used during surgery.
The Westborough, Massachusetts-based company said it had not received any reports of adverse reactions to the products it is recalling but that the U.S. Food and Drug Administration has asked it to improve its sterility testing processes.
http://www.reuters.com/article/2012/10/31/us-usa-health-meningitis-ameridose-idUSBRE89U18I20121031
http://www.reuters.com/article/2012/10/31/us-usa-health-meningitis-ameridose-idUSBRE89U18I20121031
The U.S. Food and Drug Administration announced today that Ameridose, LLC, based in Westborough, Mass., is voluntarily recalling all of its unexpired products in circulation. Products from Ameridose can be identified by markings that indicate Ameridose by name or by its company logo  . A complete list of all products subject to this recall can be accessed at www.ameridose.com
. A complete list of all products subject to this recall can be accessed at www.ameridose.com  .
.
The FDA is currently conducting an inspection of Ameridose’s facility. Although this inspection is ongoing, the FDA’s preliminary findings have raised concerns about a lack of sterility assurance for products produced at and distributed by this facility. Use of non-sterile injectable products can represent a serious hazard to health that could lead to life-threatening injuries. Most products produced at and distributed by this facility are represented by Ameridose to be sterile products. Ameridose entered into a voluntary agreement with the Massachusetts Board of Registration in Pharmacy to cease all pharmacy and manufacturing operations starting on Oct. 10, 2012.
The FDA is currently conducting an inspection of Ameridose’s facility. Although this inspection is ongoing, the FDA’s preliminary findings have raised concerns about a lack of sterility assurance for products produced at and distributed by this facility. Use of non-sterile injectable products can represent a serious hazard to health that could lead to life-threatening injuries. Most products produced at and distributed by this facility are represented by Ameridose to be sterile products. Ameridose entered into a voluntary agreement with the Massachusetts Board of Registration in Pharmacy to cease all pharmacy and manufacturing operations starting on Oct. 10, 2012.
This recall is not based on reports of patients with infections associated with any of Ameridose’s products, and the agency recommended this recall out of an abundance of caution. Therefore, at this time, the FDA is also recommending that health care professionals do not need to follow up with patients who received Ameridose products. Health care professionals should stop using Ameridose products at this time, and return them to the firm.
Hospitals, clinics, health care professionals, and other customers with Ameridose products on hand should contact Ameridose at 888-820-0622 to obtain instructions on how to return products to Ameridose.
“The FDA’s top priority is to ensure that drugs are safe for the American public,” said FDA Commissioner Margaret A. Hamburg, M.D.
Together with the State of Massachusetts, the FDA commenced the current inspection of the Ameridose facility as part of the agency’s ongoing fungal meningitis outbreak investigation. Ameridose is a company sharing common management by the same parties as New England Compounding Center (NECC) of Framingham, Mass., the firm associated with compounded drugs linked to the ongoing fungal meningitis outbreak.
“Because the preliminary results of the FDA’s inspection raise concerns about the sterility assurance of Ameridose’s products, the FDA is advising health care professionals to stop using all Ameridose products and follow the recall procedures provided by the firm,” explained Janet Woodcock, M.D., director of FDA’s Center for Drug Evaluation and Research.
The FDA has identified some Ameridose products that currently appear on the critical shortage list. These products were in shortage before the Ameridose recall, but supplies may be further affected as a result of the Ameridose recall. The FDA is working with alternative manufacturers to maintain supplies of these life-saving drugs.
“The agency is taking all steps within its authority to help prevent or alleviate shortage situations and to minimize the impact this recall may have on drug supplies,” added Commissioner Hamburg.
November 30, 2012 Meningitis Recall News Update
The Framingham pharmacy linked to the fungal meningitis outbreak blamed for the deaths of dozens of pain sufferers has been told by a federal judge not to tamper with or attempt to destroy anything within the company’s walls — or even stored on personal cellphones and home computers.
My office is lead counsel on the Green v. NECC case, and there were hearings held in that case this past week. We sought for preservation of evidence and asked for permission to inspect the facilities where the recalled products were compounded.
U.S. District Court Judge F. Dennis Saylor IV today told lawyers for New England Compounding Center and its sister operation Ameridose he intends to issue an evidence-preservation order on behalf of 12 civil suits pending against one or both of the pharmacies in Boston’s federal court alone.
At a hearing this, Saylor also temporarily consolidated the 12 lawsuits for the convenience of pre-trial discovery and proceedings. Saylor is juggling just a handful of the 70 cases that have been filed federally against NECC and Ameridose nationwide, and which he noted may all be brought under one umbrella here or in another district early next year.
http://www.bostonherald.com/news/regional/view.bg?articleid=1061178004&srvc=rss
December 7, 2012: NECC Tainted Steroid Medication Update
Our firm filed a Motion to Inspect the NECC facility. The FDA issued an inspection report, and in it there are observations of the New England Compounding Facility. A number of issues will be part of the litigation, including the "Clean Room" where the recalled products were made.
Here's a virtual clean room, put together by Purdue:
Here's a virtual clean room, put together by Purdue:
Generally found in hospitals and home health care companies, the rooms are used to prepare drugs, intravenous drips, syringes, chemotherapy treatments and the like, especially those administered directly into the bloodstream—a factor that makes vital the use of a clean room and proper clean-room procedures. Concern over the rise of antibiotic-resistant pathogens has only increased the need for such expertise.
Why this matters in the New England Compounding Pharmacy litigation: Access, production, and preparation all will be analyzed to determine if this tragedy could have been prevented if the clean room was operated the right way.
Why this matters in the New England Compounding Pharmacy litigation: Access, production, and preparation all will be analyzed to determine if this tragedy could have been prevented if the clean room was operated the right way.
1/2013 NECC Recalled Steroids - Massachusetts Plans Strict Control of Compounding Pharmacies
As the fallout from this national tragedy continues, the State of Massachusetts is in the news regarding its belated plan to provide for better control of compounding pharmacies:
The laws will be among the strongest in the country, said Kevin Outterson, a law professor at Boston University and a member of the expert panel that advised the state on how to curb abuses by companies like the New England Compounding Center, the Framingham pharmacy that made the tainted drug responsible for the nationwide meningitis outbreak.
The legislation would establish strict licensing requirements for compounding sterile drugs; let the state assess fines against pharmacies that break its rules; protect whistle-blowers who work in compounding pharmacies; and reorganize the state pharmacy board to include more members who are independent of the industry and fewer who are part of it.
http://www.nytimes.com/2013/01/05/us/massachusetts-plans-stricter-control-of-compounding-pharmacies.html?_r=0
1/25/13: Judge Appoints Trustee To Oversee NECC’s Bankruptcy
A steroid produced by the Framingham-based New England Compounding Center has been linked to a fungal meningitis outbreak that’s killed 44. The company filed for Chapter 11 bankruptcy in December, saying the purpose of the filing was to establish a compensation fund for victims.
The company at first opposed an independent trustee, saying it would delay progress toward establishing the fund. Lawyers on Thursday dropped objections, saying the company did not want a battle with creditor.
The company at first opposed an independent trustee, saying it would delay progress toward establishing the fund. Lawyers on Thursday dropped objections, saying the company did not want a battle with creditor.
http://ageorgialawyer.blogspot.com/2013/01/a-judge-has-agreed-to-appoint.html
Feb. 2013 Steroid/Meningitis Infection Update
Notice to Clinicians: Continued Vigilance Urged for
Fungal Infections among Patients Who Received
Contaminated Steroid Injections
The CDC continues to receive new reports of fungal infection among patients who were given injections of contaminated methylprednisolone acetate (MPA) from the New England Compounding Center (NECC) in Framingham, Mass. Most of these recent cases have been localized spinal or paraspinal infections (e.g., epidural abscesses) in patients, although new cases of meningitis or arachnoiditis also have been reported. Because many of these new cases are among patients with minimal symptoms, CDC is re-emphasizing the recommendation for clinicians to remain vigilant for fungal infections, especially in patients with mild or even baseline symptoms, and consider evaluation with magnetic resonance imaging (MRI) if clinically warranted.
Status of Fungal Disease Outbreak
As of March 4, 2013, a total of 720 cases, which includes 48 deaths, have been reported in 20 states. Current information about the outbreak, including case counts and distribution by state, and clinician and patient guidance, is available online athttp://www.cdc.gov/hai/outbreaks/meningitis.html.
As of March 4, 2013, a total of 720 cases, which includes 48 deaths, have been reported in 20 states. Current information about the outbreak, including case counts and distribution by state, and clinician and patient guidance, is available online athttp://www.cdc.gov/hai/outbreaks/meningitis.html.
Over the past several months, there has been a marked decrease in reports of fungal meningitis, but CDC continues to receive reports of localized spinal and paraspinal infections, which include epidural abscess, phlegmon, arachnoiditis, and discitis. Additionally, some of these newly identified case-patients had initially tested negative for signs of a fungal infection (either by lumbar puncture or MRI) and have subsequently developed fungal infection, indicating a prolonged incubation period.
After the recall of NECC steroid medications on September 26, state and local health departments identified almost 14,000 people in 23 states who were potentially exposed to the implicated MPA; of these, an estimated 11,000 individuals received spinal or paraspinal injections. Through active notification by clinics with assistance from states and CDC in early October, nearly all of these exposed persons were contacted at least once and informed of their risk for fungal infection as a result of receiving injections with contaminated medication.
Despite this and subsequent patient outreach efforts, CDC and public health partners remain concerned about the potential for some exposed patients to have localized fungal infections that have gone unrecognized. These infections may be unrecognized because some patients have not continued to receive close clinical follow-up or because they have not recognized symptoms suggestive of a localized infection, which may be difficult to distinguish from their baseline chronic pain.
As described in CDC’s HAN update on December 20(http://emergency.cdc.gov/HAN/han00338.asp), MRI testing was done on 128 patients in Michigan, Tennessee, and North Carolina who had no previous evidence of infection and had new or worsening symptoms at or near the site of their spinal or paraspinal injection. Of these, 67 (52%) had findings suggestive of localized infection. In addition, of 109 different patients reporting persistent but baseline symptoms at or near the site of their spinal or paraspinal injection, 15 (14%) also had abnormal MRI findings suggestive of infection, and 27 (25%) had non-specific enhancement of soft tissue or other paraspinal structures. The clinical significance of these findings is unclear; however, there is a theoretical risk that failure to diagnose these infections in a timely fashion could result in poor outcomes for patients (e.g., neurologic compromise, osteomyelitis, or progression to meningitis)
Patient and Clinician Recommendations
Early in the outbreak, CDC advised clinicians to closely monitor and evaluate patients who received injections of implicated MPA. Additional guidance was provided in HAN updates issued on November 20 (http://emergency.cdc.gov/HAN/han00335.asp) and December 20 (http://emergency.cdc.gov/HAN/han00338.asp). Because of the possibility that some patients may have unrecognized, localized fungal infections, CDC is re-emphasizing the following recommendations for patients who received a spinal or paraspinal injection with implicated MPA:
Patients
Patients who received an injection in or near their spine from one of the three implicated lots of MPA1 and who have any symptoms at or near the site of their injection should seek evaluation by their medical provider for the possibility of a localized infection, such as an epidural abscess. This includes patients who initially received steroid injections for pain and continue to have persistent baseline pain.
Clinicians
As a part of continued monitoring of patients who received an injection with implicated MPA, clinicians should consider re-evaluating patients who received a spinal or paraspinal injection with implicated MPA for signs and symptoms suggestive of infection, including any symptoms at or near the site of their injection. Because of the prolonged incubation period for these infections, this guidance pertains both to patients who have not been previously evaluated and to those who have already had a prior negative evaluation (e.g., normal cerebrospinal fluid profile, normal findings on MRI) but continue to have complaints:
- In patients with new or worsening symptoms at or near the site of their injection, clinicians should obtain an MRI with contrast of the symptomatic area(s).
- In patients with persistent but baseline symptoms, clinicians should consider obtaining an MRI with contrast of the symptomatic area(s) because the presentation of spinal or paraspinal infections can be subtle, and may be difficult to distinguish from a patient’s baseline chronic pain.
- In some cases, radiologic evidence of abscess or phlegmon has become apparent on repeat MRI studies performed subsequent to an initially normal imaging procedure. Clinicians should therefore have a low threshold for repeat MRI studies in patients who continue to have symptoms localizing to the site of injection, even after a normal study. However, the optimal duration between MRI studies is unknown.
- Clinicians should also consider reviewing MRI results with a neuroradiologist because of potential difficulties in interpreting imaging results for these patients.
Revised Clinical Guidance and Clinician Information Call
In response to input from expert consultants on fungal disease and physicians who have been treating patients affected by this outbreak, CDC has revised its Interim Treatment and Diagnostic Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products(http://www.cdc.gov/hai/outbreaks/clinicians/guidance_cns.html). The revisions include addition of new information on several topics, including:
- Surgical management of parameningeal disease
- Duration of antifungal treatment
- Monitoring clinical status after cessation of antifungal treatment
- Information on non-first-line medications (e.g., posaconazole or itraconazole)