The steroid medication suspected in an outbreak of a rare kind of meningitis was shipped to 23 states, according to the Centers for Disease Control. So far there are reports that a pharmacy that made the steroid, New England Compounding Center of Framingham. There were three recalled three lots of the drug last week and the company has closed.
Facilities that have received and pulled from use the potentially contaminated steroid product include :
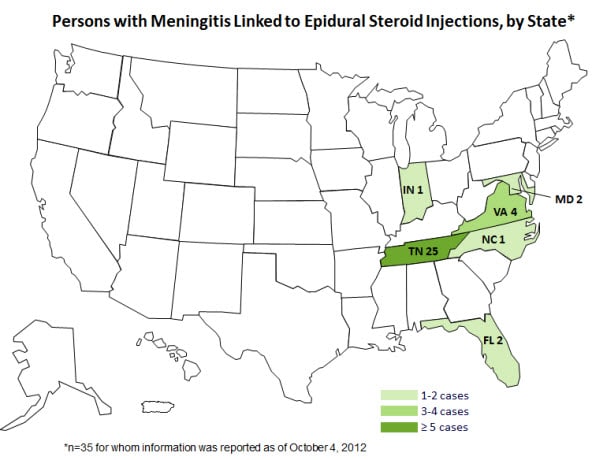
Berlin Interventional Pain Management, Berlin, MDHere is an image of cases by state (Source: http://www.enewspf.com/latest-news/health-and-fitness/37178-cdc-reports-multi-state-meningitis-outbreak-oct-4-2012.html)
Box Hill Surgery Center, Abingdon, MD
Greenspring Surgery Center, Baltimore, MD
Harford County Ambulatory Surgery Center, Edgewood, MD
Maryland Pain Specialists, Towson, MD
SurgCenter of Bel Air, Bel Air, MD Zion Ambulatory Center, Baltimore, MD
What is meningitis:
Meningitis is an inflammation of the membranes (meninges) surrounding your brain and spinal cord, usually due to the spread of an infection. The swelling associated with meningitis often triggers the "hallmark" signs and symptoms of this condition, including headache, fever and a stiff neck in anyone over the age of 2.
Here's a letter from 2006 involving this same company and its compounding:
On September 23, 2004, investigators from the U.S. Food and Drug Administration (FDA) and the Massachusetts Board of Pharmacy inspected your firm, located at 697 Waverly Street, Framingham, Massachusetts. On January 19, 2005, the inspection was completed. This inspection revealed that your firm compounds human prescription drugs in various dosage forms and strengths.
We acknowledge the receipt of your October 1, 2004, letter addressed to FDA's New England District Office, concerning questions presented during the referenced inspection.
FDA's position is that the Federal Food, Drug, and Cosmetic Act (FDCA) establishes agency jurisdiction over "new drugs," including compounded drugs. FDA's view that compounded drugs are "new drugs" within the meaning of 21 U.S.C. § 321(p), because they are not "generally recognized, among experts . . . as safe and effective," is supported by substantial judicial authority. See Weinberger v. Hynson, Westcott & Dunning, 412 U.S. 609, 619, 629-30 (1973) (explaining the definition of "new drug"); Prof'ls & Patients for Customized Care v. Shalala, 56 F.3d 592, 593 n.3 (5th Cir. 1995) (the FDCA does not expressly exempt pharmacies or compounded drugs from its new drug provisions); In the Matter of Establishment Inspection of: Wedgewood Village Pharmacy, 270 F. Supp. 2d 525, 543-44 (D.N.J. 2003), aff'd, Wedgewood Village Pharmacy v. United States, 421 F.3d 263, 269 (3d Cir. 2005) ("The FDCA contains provisions with explicit exemptions from the new drug . . . provisions. Neither pharmacies nor compounded drugs are expressly exempted."). FDA maintains that, because they are "new drugs" under the FDCA, compounded drugs may not be introduced into interstate commerce without FDA approval.
The drugs that pharmacists compound are not FDA-approved and lack an FDA finding of safety and efficacy. However, FDA has long recognized the important public health function served by traditional pharmacy compounding. FDA regards traditional compounding as the extemporaneous combining, mixing, or altering of ingredients by a pharmacist in response to a physician's prescription to create a medication tailored to the specialized needs of an individual patient . See Thompson v. Western States Medical Center, 535 U.S. 357, 360-61 (2002). Traditional compounding typically is used to prepare medications that are not available commercially, such as a drug for a patient who is allergic to an ingredient in a mass-produced product, or diluted dosages for children.
Through the exercise of enforcement discretion, FDA historically has not taken enforcement actions against pharmacies engaged in traditional pharmacy compounding. Rather, FDA has directed its enforcement resources against establishments whose activities raise the kinds of concerns normally associated with a drug manufacturer and whose compounding practices result in significant violations of the new drug, adulteration, or misbranding provisions of the FDCA.
FDA's current enforcement policy with respect to pharmacy compounding is articulated in Compliance Policy Guide (CPG), section 460.200 ["Pharmacy Compounding"], issued by FDA on May 29, 2002 (see Notice of Availability, 67 Fed. Reg. 39,409 (June 7, 2002)).1 The CPG identifies factors that the Agency considers in deciding whether to initiate enforcement action with respect to compounding. These factors help differentiate the traditional practice of pharmacy compounding from the manufacture of unapproved new drugs. They further address compounding practices that result in significant violations of the new drug, adulteration, or misbranding provisions of the FDCA. These factors include considering whether a firm compounds drugs that are copies or essentially copies of commercially available FDA-approved drug products without an FDA sanctioned investigational new drug application (IND). The factors in the CPG are not intended to be exhaustive and other factors may also be appropriate for consideration.