Worth a read, a case involving Starbucks, a stolen laptop and ID Theft. Hollow win for the Plaintiff.
The Ninth Circuit noted that under Washington law, "actual loss or damage is an essential element" of a negligence claim. The Court noted that plaintiffs waived the argument that "anxiety constitutes actionable injury.
Find the decision here: http://www.scribd.com/doc/45307040/Krottner-v-Starbucks-No-09-35823-9th-Cir-Dec-14-2010-Opinion
"Although we have not previously determined whether an increased risk of identity theft constitutes an injury-in-fact, we have addressed future harm in other contexts, holding that “the possibility of future injury may be sufficient to confer standing on plaintiffs; threatened injury constitutes ‘injury in fact.’ ” Cent. Delta Water Agency, 306 F.3d at 947. More specifically, [a] plaintiff may allege a future injury in order to comply with [the injury-in-fact] requirement, but only if he or she “is immediately in danger of sustaining some direct injury as the result of the challenged . . . conduct and the injury or threat of injury is both real and immediate, not conjectural or hypothetical."
News, musings and commentary on dietary supplements & pharmaceutical law issues, technology, and litigation. Lawyers for consumers and injured people.(No advice on this blog, though) mark(at)markzamora.com
Thursday, December 16, 2010
FDA Says More Scrutiny Over Dietary Supplements
 Image via WikipediaThe FDA took new steps aimed at keeping consumers safe from harmful products that are marketed as dietary supplements and that contain undeclared or deceptively labeled ingredients. The FDA has found that these products are often promoted for weight loss, sexual enhancement, and bodybuilding.
Image via WikipediaThe FDA took new steps aimed at keeping consumers safe from harmful products that are marketed as dietary supplements and that contain undeclared or deceptively labeled ingredients. The FDA has found that these products are often promoted for weight loss, sexual enhancement, and bodybuilding.From the FDA site:
Among the substances found in products that are marketed as dietary supplements and that contain hidden or deceptively labeled ingredients are
- the active ingredients in FDA-approved drugs or their analogs (closely-related drugs).
- other compounds, such as novel synthetic steroids, that do not qualify as dietary ingredients.
FDA has also alerted consumers to hundreds of products with these often deceptively labeled and harmful ingredients, including more than 80 products marketed for sexual enhancement, more than 70 products marketed for weight loss, and more than 80 products marketed for bodybuilding.
Interesting: We've been working on cases involving unsafe supplements for several years, and to me the problem is getting worse, not better. Let's see how the FDA moves on this.
Tuesday, December 14, 2010
Florida Law: Appellate Court Reverses Trial Court Over Discovery Limits
An interesting decision from the Florida 4th DCA. It goes to show what lengths a corporate defendant will go to keep information from being disclosed (in this case, four years of 'protracted' fights). From the appellate opinion:
It is said in this case that a trial judge unreasonably curtailed one party's pretrial discovery of directly relevant, valid and reliable information reasonably calculated to lead to admissible evidence. The argument is that the critical information about the subject matter was lodged solely with the adverse party, but the court's errant discovery restrictions resulted in one-sided evidence before the jury. Hence, there was a verdict based on faulty, defective information. It is contended that the resulting judgment must be reversed and the case returned to the trial court to allow the full measure of discovery in accord with the principles and purposes of the rules governing that process.
The underlying action was based on a personal wreck involving a fatality. The Plaintiff claimed that a tire tread separation caused a wreck, and Cooper Tire was sued. The principal issue for discovery centered on the issue of whether the tire was defective and unreasonably dangerous in ordinary operation. Equally critical to discovery's scope was Cooper Tire's "state-of-the-art" affirmative defense.
When certain documents were sought, the Defendant filed numerous motions to keep the discovery out of the hands of the Plaintiff. Typical, you say. In this case, there was at least one evidentiary hearing that last four days, and there were 'protracted hearing over the span of four years." This fight continued over the course of two judges assigned to the action.
I reversing the lower court, the 4th DCA wrote this critical explanation that discovery should have been permitted on the issue of tire tread separation:
It is difficult to comprehend any valid basis for concluding that it could not possibly have affected the jury's consideration of the claims and defenses. This was evidence from Cooper Tire's own design and manufacturing processes of all its models of passenger-light truck tires. What might be thought by reasonable jurors as merely an anomaly if found in only a single model tire quite reasonably could be seen as strong evidence of design defects if found in tires across the spectrum of the kind of product at issue. Denying plaintiff the discovery of this evidence from Cooper Tire itself is the equivalent of denying Cooper Tire discovery from plaintiff as to the use of seatbelts and maintenance of the tires, as well as of the financial and emotional effects of the death of the decedent on the members of his family.
A good read not only for the rules regarding discovery, but for the lengths a party will to to thwart the very purpose of the rules.
Find the case here: http://scholar.google.com/scholar_case?case=16653302306530515981&hl=en&as_sdt=2&as_vis=1&oi=scholarr
It is said in this case that a trial judge unreasonably curtailed one party's pretrial discovery of directly relevant, valid and reliable information reasonably calculated to lead to admissible evidence. The argument is that the critical information about the subject matter was lodged solely with the adverse party, but the court's errant discovery restrictions resulted in one-sided evidence before the jury. Hence, there was a verdict based on faulty, defective information. It is contended that the resulting judgment must be reversed and the case returned to the trial court to allow the full measure of discovery in accord with the principles and purposes of the rules governing that process.
The underlying action was based on a personal wreck involving a fatality. The Plaintiff claimed that a tire tread separation caused a wreck, and Cooper Tire was sued. The principal issue for discovery centered on the issue of whether the tire was defective and unreasonably dangerous in ordinary operation. Equally critical to discovery's scope was Cooper Tire's "state-of-the-art" affirmative defense.
When certain documents were sought, the Defendant filed numerous motions to keep the discovery out of the hands of the Plaintiff. Typical, you say. In this case, there was at least one evidentiary hearing that last four days, and there were 'protracted hearing over the span of four years." This fight continued over the course of two judges assigned to the action.
I reversing the lower court, the 4th DCA wrote this critical explanation that discovery should have been permitted on the issue of tire tread separation:
It is difficult to comprehend any valid basis for concluding that it could not possibly have affected the jury's consideration of the claims and defenses. This was evidence from Cooper Tire's own design and manufacturing processes of all its models of passenger-light truck tires. What might be thought by reasonable jurors as merely an anomaly if found in only a single model tire quite reasonably could be seen as strong evidence of design defects if found in tires across the spectrum of the kind of product at issue. Denying plaintiff the discovery of this evidence from Cooper Tire itself is the equivalent of denying Cooper Tire discovery from plaintiff as to the use of seatbelts and maintenance of the tires, as well as of the financial and emotional effects of the death of the decedent on the members of his family.
A good read not only for the rules regarding discovery, but for the lengths a party will to to thwart the very purpose of the rules.
Find the case here: http://scholar.google.com/scholar_case?case=16653302306530515981&hl=en&as_sdt=2&as_vis=1&oi=scholarr
FDA takes a Look at Mercury in Tooth Fillings
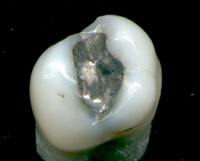 Image via WikipediaFour consumer advocacy organizations have challenged the FDA's March 2009 ruling about mercury fillings or amalgams. From 2009 on the FDA site:
Image via WikipediaFour consumer advocacy organizations have challenged the FDA's March 2009 ruling about mercury fillings or amalgams. From 2009 on the FDA site:The U.S. Food and Drug Administration today issued a final regulation classifying dental amalgam and its component parts – elemental mercury and a powder alloy—used in dental fillings. While elemental mercury has been associated with adverse health effects at high exposures, the levels released by dental amalgam fillings are not high enough to cause harm in patients.
The regulation classifies dental amalgam into Class II (moderate risk). By classifying a device into Class II, the FDA can impose special controls (in addition to general controls such as good manufacturing practices that apply to all medical devices regardless of risk) to provide reasonable assurance of the safety and effectiveness of the device.
The special controls that the FDA is imposing on dental amalgam are contained in a guidance document that contains, among other things, recommendations on performance testing, device composition, and labeling statements.
Specifically, the FDA recommended that the product labeling include:
- A warning against the use of dental amalgam in patients with mercury allergy;
- A warning that dental professionals use adequate ventilation when handling dental amalgam;
- A statement discussing the scientific evidence on the benefits and risk of dental amalgam, including the risks of inhaled mercury vapor. The statement will help dentists and patients make informed decisions about the use of dental amalgam.
The advocacy groups cite new studies pointing to neurological conditions and even Alzheimer's disease as consequences of some people's dental work. The organizations are asking the FDA's dental-products panel to, at the very least, reclassify the amalgam to bar its use in pregnant women and children. According to the news reports, "groups are also accusing the FDA and the American Dental Association of maintaining too cozy a relationship with the dental products industry."
Interesting reading from newsday, where you can find the article: http://www.newsday.com/long-island/fda-revisits-use-of-mercury-in-tooth-fillings-1.2540068
Friday, December 10, 2010
More Good News about Amazing Aspirin
 From NPR:
From NPR:I'm one of those guys who takes an aspirin daily. So, I read this news with interest:
A British study claims evidence that aspirin can prevent death from a variety of cancers, if you take the medicine long enough in middle age. Even a baby aspirin might be enough.
Published in The Lancet, the study suggests aspirin could provide protection against a wide variety of tumors. They range from pancreatic cancer to the type of lung cancer that strikes nonsmokers.
For some of these cancers, the reduction in death was noteworthy. Twenty years after people had started taking aspirin regularly (and kept it up for at least five years), their deaths from esophageal cancer were reduced by 60 percent compared to study subjects who got a placebo.
http://www.npr.org/blogs/health/2010/12/07/131873139/study-aspirin-cuts-deaths-from-wide-variety-of-cancers
Read more:
Levaquine: Verdict for Plaintiff in first trial -Jury's Discussion Point? Responsibility
 Image by Ricky Romero via FlickrYesterday a jury in Minneapolis today awarded a verdict in the case of John Schedin, who sued J&J and its Ortho-McNeil-Janssen Pharmaceuticals unit in 2008. Schedin said he ruptured both Achilles tendons after taking Levaquin. He claimed the companies failed to warn doctors and patients of the drug’s association with tendon damage.
Image by Ricky Romero via FlickrYesterday a jury in Minneapolis today awarded a verdict in the case of John Schedin, who sued J&J and its Ortho-McNeil-Janssen Pharmaceuticals unit in 2008. Schedin said he ruptured both Achilles tendons after taking Levaquin. He claimed the companies failed to warn doctors and patients of the drug’s association with tendon damage.This is the first case of more than 2,600 claims in U.S. courts alleging that Levaquin caused tendon damage in patients and that J&J failed to disclose the risk adequately.
In a report, particularly telling is a juror's statement: “We talked a lot about the responsibility the company had to the general public, as far as safety goes,” Zach Rawson, a juror from Rochester, Minnesota, said after the trial. “ We felt that they didn’t warn adequately, that they didn’t use enough means of warning the public, especially the doctors.”
Source: http://www.bloomberg.com/news/2010-12-08/johnson-johnson-must-pay-1-1-million-in-punitive-damages-jury-says.html
Vanity Fair's Must Read: Deadly Medicine
 Image by higlu via FlickrA gripping article in Vanity Fair this month take a look at the outsourcing of clinical trials for proposed prescription drugs.
Image by higlu via FlickrA gripping article in Vanity Fair this month take a look at the outsourcing of clinical trials for proposed prescription drugs.It's an eye opener. "Many of today’s trials still take place in developed countries, such as Britain, Italy, and Japan. But thousands are taking place in countries with large concentrations of poor, often illiterate people, who in some cases sign consent forms with a thumbprint, or scratch an “X.”
Since the first week of posts on this blog, I have been critical of the FDA and many of the rules, regulations, and efforts by this guardian of the citizens to keep big pharma companies working in a safer way. The FDA fails people like you and me. An excerpt:
One big factor in the shift of clinical trials to foreign countries is a loophole in F.D.A. regulations: if studies in the United States suggest that a drug has no benefit, trials from abroad can often be used in their stead to secure F.D.A. approval. There’s even a term for countries that have shown themselves to be especially amenable when drug companies need positive data fast: they’re called “rescue countries.”
This article - a 21st century version, in shorter form, of The Jungle.
Read it here: http://www.vanityfair.com/politics/features/2011/01/deadly-medicine-201101
Tuesday, December 07, 2010
December 7, 2010 Darvocet Legal Recall News and Updates
Some folks are asking why did it take 30 years to pull Darvocet/Darvon off the market? As Dr. Sidney Wolfe, a drug expert at Public Citizen in Washington, D.C.,has said to many reporters: "Propoxyphene has been one of the top 50-selling drugs for at least 40 years," and its sales have generated huge profits for drug companies. Yet its dangers have been well known for most of that time.
Estimates are that several thousand people died as a result of taking the drug, countless others developed serious medical problems, and millions wasted money on an expensive painkiller that didn't work.
Over the years, many experts called for the removal of propoxyphene, but those calls were routinely ignored. Public Citizen petitioned the FDA to ban the drug in 1978 and again in 2006; finally, the group sued the FDA in 2008.
From the BBC in 2004: A popular painkiller is being withdrawn from the UK market over concerns about links with suicide. Co-proxamol, used by thousands for conditions such as back pain, will be phased out over the next year or two. Data showed fatal overdoses due to co-proxamol are the second most frequent means of suicide with prescribed drugs in England and Wales, accounting for up to 400 deaths each year.The risk of death associated with co-proxamol overdose seems to be higher than for either tricyclic antidepressants or paracetamol. http://news.bbc.co.uk/2/hi/health/4221829.stm
Read more: http://www.sacbee.com/2010/12/05/3229146/pain-drug-propoxyphene-should.html#ixzz17PgVpHLH
Read more: http://www.sacbee.com/2010/12/05/3229146/pain-drug-propoxyphene-should.html#ixzz17Pg2ZvtN
Estimates are that several thousand people died as a result of taking the drug, countless others developed serious medical problems, and millions wasted money on an expensive painkiller that didn't work.
Over the years, many experts called for the removal of propoxyphene, but those calls were routinely ignored. Public Citizen petitioned the FDA to ban the drug in 1978 and again in 2006; finally, the group sued the FDA in 2008.
Six years ago, the drug was banned in the United Kingdom based on data that it didn't work and, at doses only slightly above those often prescribed, leads to accidental overdoses.
In 2004, British health authorities ordered a phased withdrawal of the drug, known in the U.K. as co-proxamol. It was withdrawn gradually rather than immediately because the narcotic has addictive properties and patients require adequate time to switch to other painkillers.
According to British health authorities, the drug was banned because “each year there are 300- 400 fatalities following deliberate or accidental drug overdose involving propoxyphene/acetaminophen in England and Wales alone. Approximately one-fifth [60-80] of these deaths are considered to be accidental.” The British government further stated that the drug’s effectiveness “is poorly established and the risk of toxicity in overdose, both accidental and deliberate, is unacceptable.... It has not been possible to identify any patient group in whom the risk-benefit [ratio] may be positive.” http://healthfully.org/dnd/id8.html
Finally in 2008 the UK banned it outright. Interestingly, the UK's role seemed to be to reduce suicides, as opposed to concerns about heart conditions.
Read more here: http://blogs.wsj.com/health/2010/11/24/without-darvon-and-darvocet-whats-a-pain-sufferer-to-do/
Read more: http://www.sacbee.com/2010/12/05/3229146/pain-drug-propoxyphene-should.html#ixzz17PgVpHLH
Read more: http://www.sacbee.com/2010/12/05/3229146/pain-drug-propoxyphene-should.html#ixzz17Pg2ZvtN
Monday, December 06, 2010
Prempro: Verdict for the Defendant
Wyeth properly warned a Virginia woman’s doctors about the risks of its Prempro menopause drug, a jury ruled today in rejecting her claim for damages. The Plaintiff was Ms. Torkie -Tork.
The verdict was reached in USDCT in Alexandria, Virginia last week. It took just a few hours to reach the result. Wyeth has now successfully defended four lawsuits in a row.
Also last week the Nevada Supreme Court last week affirmed a $57.6 million award to three women who said Prempro was the cause of the cancer each suffered. The verdict included $35 million in punitive damages the panel handed down in 2007 over Wyeth officials’ mishandling of the drug.
Source: http://www.bloomberg.com/news/2010-12-03/pfizer-properly-warned-about-prempro-health-risks-jury-finds.html?cmpid=yhoo
The verdict was reached in USDCT in Alexandria, Virginia last week. It took just a few hours to reach the result. Wyeth has now successfully defended four lawsuits in a row.
Also last week the Nevada Supreme Court last week affirmed a $57.6 million award to three women who said Prempro was the cause of the cancer each suffered. The verdict included $35 million in punitive damages the panel handed down in 2007 over Wyeth officials’ mishandling of the drug.
Source: http://www.bloomberg.com/news/2010-12-03/pfizer-properly-warned-about-prempro-health-risks-jury-finds.html?cmpid=yhoo
Sunday, December 05, 2010
Google Earth: Using it as Demonstrative Evidence
 Found this from another lawyer on the web:
Found this from another lawyer on the web:State ex rel J.B., Case No. A-2228-08T4 (N.J. Ct. App.; Sept. 27, 2010)
Some key language in the opinion:
The [c]ourt finds that other than very recently what would have happened is . . . that the State would have brought in an atlas map and ask[ed] somebody familiar with the area to point on the map where different locations are and how you would get there. And this is just an updated manner of getting the same information. If the [d]efense wants to show that the information is incorrect, they can certainly do it by either cross examination or they can do exactly what I just suggested and bring in an atlas map and show where the exhibit that the State is offering is incorrect.
Pretty sound reasoning in my view.
Saturday, December 04, 2010
Keeping an Eye on Lupron News
Lupron As Hormone Therapy
Abbott Labs is the marker of Lupron, more commonly referred to as leuprolide. Lupron is supposed to help both men and women's sexual health, ailing in the treatment of prostate cancer for men and helping to combat endometriosis in women. The medication may also be used for early puberty.There are five types of Lupron that can be injected under a patients skin to help with his or her hormone therapy. The kinds of Lupron are:
- Lupron Depot 30
- Lupron Depot 22.5
- Lupron Depot 7.5
- Lupron Depot 11.25
- Lupron Depot 3.75
There is a long list of potential side effects linked to Lupron. Some apply only to men and others affect just women, but others, such as chronic muscle pain, have been reported in both sexes.
Lupron side effects that have been documented are:
- Chronic muscle pain and weakness
- Back pain
- Joint pain
- Limb pain
- Urine with blood in it
- Diarrhea
- Severe headaches
- Vomiting
- Muscle atrophy
- Depression
- Decreasing of hemoglobin
- Decreasing of hematocrit
- Bone density changes
- Loss of the sense of smell
- Loss of the sense of taste
- Enlargement and sensitivity to breasts (women)
- Decrease in size of testicles (men)
- Erectile dysfunction (men)
- Decreased sexual desire (both men and women)
- Thyroid problems
The U.S. Food and Drug Administration (FDA) has notified the manufacturers of the Gonadotropin-Releasing Hormone (GnRH) agonists of the need to add new safety information to the Warnings and Precautions section of the drug labels. This new information warns about increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke) in men receiving these medications for the treatment of prostate cancer. FDA’s notification to manufacturers of GnRH agonists to add this safety information is based on the Agency’s review of several published studies1-7, described in the Agency’s Ongoing Safety Review of GnRH Agonists and possible increased risk of diabetes and certain cardiovascular diseases, issued in May 2010.
GnRH agonists are approved to treat the symptoms (palliative treatment) of advanced prostate cancer. The benefits of GnRH agonist use for earlier stages of prostate cancer that have not spread (non-metastatic prostate cancer) have not been established.
Although the risk for diabetes and cardiovascular diseases appears to be low in men receiving GnRH agonists for prostate cancer, it is important for healthcare professionals to evaluate patients for risk factors for these diseases. Healthcare professionals should always carefully weigh the benefits and risks of using GnRH agonists before determining appropriate treatment for prostate cancer.
Patients who are receiving treatment with GnRH agonists should undergo periodic monitoring of blood glucose and/or glycosylated hemoglobin (HbA1c). Increased blood glucose levels may represent development of diabetes or worsening of blood glucose control in patients with diabetes. Healthcare professionals should also monitor patients for signs and symptoms suggestive of development of cardiovascular disease and manage according to current clinical practice.
Additional Information for Patients
- GnRH agonists are sold as the brand names – Lupron, Zoladex, Trelstar, Viadur, and Eligard.
- Before receiving GnRH agonists, tell your healthcare professional if you have diabetes, heart disease, a previous heart attack or stroke, or any cardiovascular risk factors like high blood pressure, high cholesterol, or cigarette smoking.
- If you have any concerns about receiving these medicines, talk to your healthcare professional.
- Report any side effects from the use of GnRH agonists to the FDA MedWatch program, using the information in the "Contact Us" box at the bottom of the page.
Thursday, December 02, 2010
Drug Induced Liver Injuries from Anti-microbial Medications
This month there will be a report released in Hepatology journal that suggest that anti-microbial medications are a common cause of drug-induced liver injury (DILI) leading to acute liver failure or ALF. Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease (such as jaundice), and indicates that the liver has sustained severe damage (loss of function of 80-90% of liver cells). The complications are hepatic encephalopathy and impaired protein synthesis (as measured by the levels of serum albumin and the prothrombin time in the blood). Source.
What could be the cause? More than 1100 drugs, herbal remedies, natural products, vitamins, minerals, and dietary supplements are known to cause liver injury.
(You may find the abstract here: http://onlinelibrary.wiley.com/doi/10.1002/hep.23937/abstract)
Detailed case reports were collected from 1,198 subjects with ALF enrolled at 23 sites participating in the National Institutes of Health-funded Acute Liver Failure Study Group. The findings included evidence that showed high female predominance in DILI ALF, suggesting that women may be more susceptible to liver injury or use more prescription drugs than men.
What were the types of drugs and products? More than sixty, alone or in combination, could cause liver injury and failure in the study population. Anti-microbial agents were found to be the most common cause of DILI ALF cases and included anti-tuberculosis drugs (25), sulphur-containing drugs (12), nitrofurantoin (12), other antibiotics (7), antifungal agents (6), and anti-retroviral drugs (4).
So, what type of drugs/brand names might make this sub group of 60? Examples of drugs that can cause acute liver hepatitis include acetaminophen (Tylenol), phenytoin (Dilantin), aspirin, isoniazid (Nydrazid, Laniazid), diclofenac (Voltaren), and amoxicillin/clavulanic acid (Augmentin). Examples of drugs that can cause chronic hepatitis include minocycline (Minocin), nitrofurantoin (Furadantin, Macrodantin), phenytoin (Dilantin), propylthiouracil, and fenofibrate (Tricor). The Merck Manual has a comprehensive list as well: http://www.merckmanuals.com/professional/sec03/ch024/ch024c.html
.
Patients who develop ALF after taking these typically did not experience a spontaneous recovery—the transplant-free survival rate in this study was 27%.
Stay tuned for the full article.
What could be the cause? More than 1100 drugs, herbal remedies, natural products, vitamins, minerals, and dietary supplements are known to cause liver injury.
(You may find the abstract here: http://onlinelibrary.wiley.com/doi/10.1002/hep.23937/abstract)
Detailed case reports were collected from 1,198 subjects with ALF enrolled at 23 sites participating in the National Institutes of Health-funded Acute Liver Failure Study Group. The findings included evidence that showed high female predominance in DILI ALF, suggesting that women may be more susceptible to liver injury or use more prescription drugs than men.
What were the types of drugs and products? More than sixty, alone or in combination, could cause liver injury and failure in the study population. Anti-microbial agents were found to be the most common cause of DILI ALF cases and included anti-tuberculosis drugs (25), sulphur-containing drugs (12), nitrofurantoin (12), other antibiotics (7), antifungal agents (6), and anti-retroviral drugs (4).
So, what type of drugs/brand names might make this sub group of 60? Examples of drugs that can cause acute liver hepatitis include acetaminophen (Tylenol), phenytoin (Dilantin), aspirin, isoniazid (Nydrazid, Laniazid), diclofenac (Voltaren), and amoxicillin/clavulanic acid (Augmentin). Examples of drugs that can cause chronic hepatitis include minocycline (Minocin), nitrofurantoin (Furadantin, Macrodantin), phenytoin (Dilantin), propylthiouracil, and fenofibrate (Tricor). The Merck Manual has a comprehensive list as well: http://www.merckmanuals.com/professional/sec03/ch024/ch024c.html
.
Patients who develop ALF after taking these typically did not experience a spontaneous recovery—the transplant-free survival rate in this study was 27%.
Stay tuned for the full article.
Wednesday, December 01, 2010
12/1/10 Darvocet Legal: FDA Drug Safety Podcast for Healthcare Professionals: FDA recommends against the continued use of propoxyphen
Good stuff in this transcript from the FDA's podcast:
Welcome, my name is Mary Kremzner, a pharmacist in the Division of Drug Information. On November 19, 2010, the Food and Drug Administration issued a Drug Safety Communication recommending against continued prescribing and use of the pain reliever propoxyphene because new data show that the drug can cause serious toxicity to the heart, even when used at therapeutic doses. FDA has requested that companies voluntarily withdraw propoxyphene from the United States market.
Propoxyphene is an opioid pain reliever used to treat mild to moderate pain. It is sold under various names as a single-ingredient product, for example Darvon, and as part of a combination product with acetaminophen, named Darvocet.
FDA's recommendation is based on all available data including data from a new study that evaluated the effects that increasing doses of propoxyphene have on the heart. The results of the new study showed that when propoxyphene was taken at therapeutic doses, there were significant changes to the electrical activity of the heart: prolonged PR interval, widened QRS complex and prolonged QT interval. These changes, which can be seen on an electrocardiogram, or EKG, can increase the risk for serious abnormal heart rhythms. FDA has concluded that the safety risks of propoxyphene outweigh its benefits for pain relief at recommended doses.
In July 2009, FDA announced an ongoing safety review of propoxyphene, which included evaluating its potential effects on the heart.
Following receipt of a Citizen Petition requesting the withdrawal of propoxyphene-containing products from the United States market, FDA convened an Advisory Committee meeting on January 30, 2009. After presentations by FDA, the petitioner, and the company reviewing the efficacy and safety data from the propoxyphene drug applications, the literature and postmarketing safety databases, the committee voted by a narrow margin of 14-to-12 against the continued marketing of propoxyphene products. Those who voted for propoxyphene to remain on the market advised requiring improved labeling, particularly with warnings about use in elderly patients and about use with concomitant opioids or alcohol. Finally, there was general agreement that additional information about the cardiac effects of propoxyphene would be relevant in further weighing the risk and benefit. As a result, under new authorities given to FDA by the Food and Drug Administration Amendments Act, the agency required the drug manufacturer to conduct a thorough QT study to formally evaluate the effects of propoxyphene on cardiac electrophysiology. In order to determine a safe supratherapeutic dose to incorporate into the Thorough QT study, FDA required the drug manufacturer to first conduct a multiple-ascending dose study or MAD.
The MAD study was a randomized, double-blind, placebo-controlled sequential multiple-ascending dose study of propoxyphene for 11 days. The study was conducted in healthy volunteers. The first cohort of study subjects was dosed with a total daily dose of 600 mg of propoxyphene (the maximum labeled dose) and the second cohort was dosed with a total daily dose of 900 mg. Additional doses were planned, however the study was placed on clinical hold due to safety concerns. Study subjects were monitored with telemetry and intermittent EKG recordings, comparable to the monitoring that would occur during a Thorough QT study.
The drug manufacturer has submitted to FDA the results from the 600 mg and 900 mg cohorts.
Significant QTc interval prolongations were observed with the propoxyphene 600 mg and 900 mg dose levels. With the 600 mg daily dose, at steady state on Treatment Day 11, the largest mean change of QTc was 29.8 milliseconds, which occurred 7 hours after the last dose; with the 900 mg dose the largest mean change was 38.2 milliseconds, which occurred 2 hours after the last dose. It is recognized in the International Conference on Harmonisation, or ICH, E14 Guideline1 that drugs that prolong the mean QT/QTc interval by >20 milliseconds have a substantially increased likelihood of being proarrhythmic. In addition, a dose-dependent prolongation of PR and QRS intervals was observed in the study.
Because the elderly and patients with renal insufficiency have a reduction in the clearance of propoxyphene and its cardioactive metabolite, norpropoxyphene, through the kidneys, these populations can be especially susceptible to proarrhythmic effects of the drug.
At this time, FDA recommends that Healthcare Professionals:
Welcome, my name is Mary Kremzner, a pharmacist in the Division of Drug Information. On November 19, 2010, the Food and Drug Administration issued a Drug Safety Communication recommending against continued prescribing and use of the pain reliever propoxyphene because new data show that the drug can cause serious toxicity to the heart, even when used at therapeutic doses. FDA has requested that companies voluntarily withdraw propoxyphene from the United States market.
Propoxyphene is an opioid pain reliever used to treat mild to moderate pain. It is sold under various names as a single-ingredient product, for example Darvon, and as part of a combination product with acetaminophen, named Darvocet.
FDA's recommendation is based on all available data including data from a new study that evaluated the effects that increasing doses of propoxyphene have on the heart. The results of the new study showed that when propoxyphene was taken at therapeutic doses, there were significant changes to the electrical activity of the heart: prolonged PR interval, widened QRS complex and prolonged QT interval. These changes, which can be seen on an electrocardiogram, or EKG, can increase the risk for serious abnormal heart rhythms. FDA has concluded that the safety risks of propoxyphene outweigh its benefits for pain relief at recommended doses.
In July 2009, FDA announced an ongoing safety review of propoxyphene, which included evaluating its potential effects on the heart.
Following receipt of a Citizen Petition requesting the withdrawal of propoxyphene-containing products from the United States market, FDA convened an Advisory Committee meeting on January 30, 2009. After presentations by FDA, the petitioner, and the company reviewing the efficacy and safety data from the propoxyphene drug applications, the literature and postmarketing safety databases, the committee voted by a narrow margin of 14-to-12 against the continued marketing of propoxyphene products. Those who voted for propoxyphene to remain on the market advised requiring improved labeling, particularly with warnings about use in elderly patients and about use with concomitant opioids or alcohol. Finally, there was general agreement that additional information about the cardiac effects of propoxyphene would be relevant in further weighing the risk and benefit. As a result, under new authorities given to FDA by the Food and Drug Administration Amendments Act, the agency required the drug manufacturer to conduct a thorough QT study to formally evaluate the effects of propoxyphene on cardiac electrophysiology. In order to determine a safe supratherapeutic dose to incorporate into the Thorough QT study, FDA required the drug manufacturer to first conduct a multiple-ascending dose study or MAD.
The MAD study was a randomized, double-blind, placebo-controlled sequential multiple-ascending dose study of propoxyphene for 11 days. The study was conducted in healthy volunteers. The first cohort of study subjects was dosed with a total daily dose of 600 mg of propoxyphene (the maximum labeled dose) and the second cohort was dosed with a total daily dose of 900 mg. Additional doses were planned, however the study was placed on clinical hold due to safety concerns. Study subjects were monitored with telemetry and intermittent EKG recordings, comparable to the monitoring that would occur during a Thorough QT study.
The drug manufacturer has submitted to FDA the results from the 600 mg and 900 mg cohorts.
Significant QTc interval prolongations were observed with the propoxyphene 600 mg and 900 mg dose levels. With the 600 mg daily dose, at steady state on Treatment Day 11, the largest mean change of QTc was 29.8 milliseconds, which occurred 7 hours after the last dose; with the 900 mg dose the largest mean change was 38.2 milliseconds, which occurred 2 hours after the last dose. It is recognized in the International Conference on Harmonisation, or ICH, E14 Guideline1 that drugs that prolong the mean QT/QTc interval by >20 milliseconds have a substantially increased likelihood of being proarrhythmic. In addition, a dose-dependent prolongation of PR and QRS intervals was observed in the study.
Because the elderly and patients with renal insufficiency have a reduction in the clearance of propoxyphene and its cardioactive metabolite, norpropoxyphene, through the kidneys, these populations can be especially susceptible to proarrhythmic effects of the drug.
At this time, FDA recommends that Healthcare Professionals:
- Stop prescribing and dispensing propoxyphene-containing products to patients.
- Contact patients currently taking propoxyphene-containing products and ask them to discontinue the drug.
- Inform patients of the risks associated with propoxyphene.
- Discuss alternative pain management strategies other than propoxyphene with your patients.
- Be aware of the possible risk of cardiac conduction abnormalities, for example prolonged QT, PR, and QRS intervals, in patients taking propoxyphene and assess patients for these events if they present with any signs or symptoms of arrhythmia.
- Report any side effects with propoxyphene to FDA's MedWatch program at www.fda.gov/medwatch.
Align Technologies and Invisalign get FDA Warning Letter
|
Tuesday, November 30, 2010
Maryland County Bans Caffine-Alcohol Drinks
 Image by Getty Images via @daylifeHoward County is banning the sale of alcoholic beverages that contain caffeine, effective at the close of business on Wednesday, Dec. 1, County Executive Kenneth Ulman and Health Officer Peter Beilenson announced Tuesday.
Image by Getty Images via @daylifeHoward County is banning the sale of alcoholic beverages that contain caffeine, effective at the close of business on Wednesday, Dec. 1, County Executive Kenneth Ulman and Health Officer Peter Beilenson announced Tuesday.County Executive Kenneth Ulman and Health Officer Dr. Peter Beilenson announced Tuesday, Nov. 30.
The ban— which applies to products such as Four Loko, Joose and Moonshot— comes after the Food and Drug Administration warning issued Nov. 17 that adding caffeine to alcoholic beverages is not recognized as safe.
Lisa de Hernandez, spokeswoman for the county Health Department, said there are about 25 different caffeine-infused alcoholic beverages that are made by four or five different companies in the United States.
“These ‘drinks’ are marketed to young adults despite the fact that one container provides the alcohol equivalent of three beers — nobody could, or should, try to consume that type of drink. We are banning them now because they are tragedies waiting to happen,” County Executive Kenneth Ulman said in a statement announcing the ban.
Beilenson agreed, noting the public health concern posed by the drinks because of the caffeine and high alcohol content.
“This combination of ingredients decreases an individual’s awareness of their alcohol intake potentially leading to serious illness or death,” he said in a statement.
Michigan and Washington state recently banned the drinks, and other states reportedly are considering bans as well. The drinks also have been banned on several college campuses.
The Howard County Health Department plans to send out letters Dec. 1 formally notifying county liquor establishments of the ban.
“Generally speaking, the turnaround time (between issuing and enforcing a ban) is not this fast,” Hernandez said, but “it’s a dangerous drink.”
“Alcohol is a depressant and caffeine is a stimulant,” she added. “You don’t feel the effects with the caffeine in there as you normally would.”
The Health Department plans to conduct follow-up investigations to make sure retailers are complying with the ban, but it encourages any resident who witnesses a sale of a alcoholic beverage containing caffeine in the county after Dec. 1 to report the incident to askhealth@howardcountymd.gov.
The first offense would result in a $50 fine, the second a $100 fine and the third a $500 fine, Hernandez said.
http://www.explorehoward.com/news/77294/howard-county-bans-controversial-alcohol-caffeine-drinks-four-loko/
Monday, November 29, 2010
November 29, 2010 Darvocet Legal News (Georgia/Florida)
Here is background and historical information from the FDA about Darvocet and Darvon:
Following receipt of a Citizen Petition requesting the withdrawal of propoxyphene-containing products from the United States market, FDA convened an Advisory Committee meeting on January 30, 2009. After presentations by FDA, the petitioner, and the company reviewing the efficacy and safety data from the propoxyphene drug applications, the literature and postmarketing safety databases, the committee voted by a narrow margin (14-to-12) against the continued marketing of propoxyphene products.
Those who voted for propoxyphene to remain on the market advised requiring improved labeling, particularly with warnings about use in elderly patients and about use with concomitant opioids or alcohol. Finally, there was general agreement that additional information about the cardiac effects of propoxyphene would be relevant in further weighing the risk and benefit. As a result, under new authorities given to FDA by the Food and Drug Administration Amendments Act (FDAAA), the agency required the drug manufacturer to conduct a thorough QT study to formally evaluate the effects of propoxyphene on cardiac electrophysiology. In order to determine a safe supratherapeutic dose to incorporate into the Thorough QT study, FDA required the drug manufacturer to first conduct a multiple-ascending dose (MAD) study.
The MAD study was a randomized, double-blind, placebo-controlled sequential multiple-ascending dose study of propoxyphene for 11 days. The study was conducted in healthy volunteers. The first cohort of study subjects was dosed with a total daily dose of 600 mg of propoxyphene (the maximum labeled dose) and the second cohort was dosed with a total daily dose of 900 mg. Additional doses were planned, however the study was placed on clinical hold due to safety concerns. Study subjects were monitored with telemetry and intermittent ECG recordings, comparable to the monitoring that would occur during a Thorough QT study.
The drug manufacturer has submitted to FDA the results from the 600 mg and 900 mg cohorts.
Significant QTc interval prolongations were observed with the propoxyphene 600 mg and 900 mg dose levels. With the 600 mg daily dose, at steady state on Treatment Day 11, the largest mean change of QTcF* (ΔΔQTcF) was 29.8 milliseconds (ms), which occurred 7 hours after the last dose; with the 900 mg dose the largest mean change was 38.2 ms, which occurred 2 hours after the last dose. It is recognized in the International Conference on Harmonisation (ICH) E14 Guideline1 that drugs that prolong the mean QT/QTc interval by >20 ms have a substantially increased likelihood of being proarrhythmic. In addition, a dose-dependent prolongation of PR and QRS intervals was observed in the study.
Because the elderly and patients with renal insufficiency have a reduction in the clearance of propoxyphene and its cardioactive metabolite, norpropoxyphene, through the kidneys, these populations can be especially susceptible to proarrhythmic effects of the drug.
FDA has concluded that the safety risks of propoxyphene outweigh its limited benefits2-6 for pain relief at recommended doses.
*QTcF is the QT interval corrected for heart rate using the Fridericia formula (cubic root – QTcF = QT/RR1/3).
References
- Guidance for Industry – E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. October 2005.
- Beaver WT. Mild analgesics. A review of their clinical pharmacology. II. Am J Med Sci. 1966;251:576-99 concl.
- Lasagna L. The clinical evaluation of morphine and its substitutes as analgesics. Pharmacol Rev. 1964;16:47-83.
- Collins SL, Edwards JE, Moore RA, McQuay HJ. Single dose dextropropoxyphene, alone and with paracetamol (acetaminophen), for postoperative pain. Cochrane Database Syst Rev. 2000;(2):CD001440.
- Collins SL, Edwards JE, Moore RA, McQuay HJ. Single-dose dextropropoxyphene in post-operative pain: a quantitative systematic review. Eur J Clin Pharmacol. 1998;54:107-12.
- Veterans Health Administration Pharmacy Benefits Management Strategic Healthcare Group and Medical Advisory Panel. Review of the Efficacy and Safety of Propoxyphene. March 2006.
Another Tylenol Recall
J and J has recalled 9.3 million bottles of Tylenol cold treatments because they don’t disclose the medicine’s alcohol content on the front label.
The recall affects three types of Tylenol Multi-Symptom liquid cold medicine sold in the U.S. by the McNeil Consumer Healthcare unit, J&J spokeswoman Bonnie Jacobs said today in a telephone interview. Flavoring agents that contain less than 1 percent alcohol are used in the formulations, and the alcohol content was listed on the package rather than the front of the bottle, according to a statement on J&J’s website.
“This is a wholesale and retail level recall and is not being undertaken on the basis of adverse events,” J&J said on the website. “No action is required by consumers or health-care providers and consumers can continue to use the product.”
Source here: http://www.bloomberg.com/news/2010-11-24/j-j-mcneil-unit-recalls-some-tylenol-cold-products-over-labeling-mistake.html?cmpid=yhoo
The recall affects three types of Tylenol Multi-Symptom liquid cold medicine sold in the U.S. by the McNeil Consumer Healthcare unit, J&J spokeswoman Bonnie Jacobs said today in a telephone interview. Flavoring agents that contain less than 1 percent alcohol are used in the formulations, and the alcohol content was listed on the package rather than the front of the bottle, according to a statement on J&J’s website.
“This is a wholesale and retail level recall and is not being undertaken on the basis of adverse events,” J&J said on the website. “No action is required by consumers or health-care providers and consumers can continue to use the product.”
Source here: http://www.bloomberg.com/news/2010-11-24/j-j-mcneil-unit-recalls-some-tylenol-cold-products-over-labeling-mistake.html?cmpid=yhoo
Saturday, November 27, 2010
Advanced Bionics Announces Voluntary Recall of the HiRes 90K
From the FDA:
Advanced Bionics (AB), a global leader in developing advanced cochlear implant systems, announced today that it has notified the US Food & Drug Administration (FDA) that it will voluntarily recall its HiRes 90K cochlear implant device and is retrieving all unimplanted devices in distribution. This action is being taken in response to two confirmed instances where the product experienced a malfunction requiring explantation. These recipients experienced severe pain, overly loud sounds and/or shocking sensations at 8-10 days after initial activation of their device.
AB is continuing to evaluate the root cause(s) of the problem and is working closely with the FDA to address their questions and concerns, and institute changes to the product to ensure that the HiRes 90K has the highest quality for patients who use the device. This voluntary action is being taken to ensure continued patient safety and product quality. The risk of any significant adverse medical events appears to be remote at present.
http://www.fda.gov/Safety/Recalls/ucm234901.htm
Advanced Bionics (AB), a global leader in developing advanced cochlear implant systems, announced today that it has notified the US Food & Drug Administration (FDA) that it will voluntarily recall its HiRes 90K cochlear implant device and is retrieving all unimplanted devices in distribution. This action is being taken in response to two confirmed instances where the product experienced a malfunction requiring explantation. These recipients experienced severe pain, overly loud sounds and/or shocking sensations at 8-10 days after initial activation of their device.
AB is continuing to evaluate the root cause(s) of the problem and is working closely with the FDA to address their questions and concerns, and institute changes to the product to ensure that the HiRes 90K has the highest quality for patients who use the device. This voluntary action is being taken to ensure continued patient safety and product quality. The risk of any significant adverse medical events appears to be remote at present.
http://www.fda.gov/Safety/Recalls/ucm234901.htm
Friday, November 26, 2010
Friday Tech: Apps to be Thankful for in 2010
 Image by jetalone via Flickr
Image by jetalone via FlickrSince opening my own law offices a couple of years ago, the great equalizer has been technology. Each year my list of Resolutions is loaded with tech-centric proposed changes. In 2010, I've converted to an iPad for hearings, and now have an Android phone to replace the iPhone. For those who read this blog, you know that I am a fan of free tech. So, here is my list of tech apps for droid and the iPad I am especially happy to use for fun and for work:
1.Avast: Avast is a free anti-virus program for computer users to protect their systems for adware, spyware and viruses floating around. Find it here: avast
2.Pandora: I'm an (aging/creaky) athlete, and I run. A lot. This is the best thing since the iPod. A company that almost went under until the iPhone arrived (and the app for it), that is now doing well. http://www.pandora.com/
3.Dropbox: Dropbox is a Web-based file hosting service operated by Dropbox, Inc. which uses cloud computing to enable users to store and share files and folders. http://www.dropbox.com/
4.isynchr: I have more than 10,000 songs in itunes, but when I bought my android HTC phone, figured that 'd have to carry two devices. Wrong. Isyncr is an add that syncs the Android phone with iTunes. The lite version is free, the full on is about two bucks.
5.Open Office: If you are starting your own office, or have simply had enough of the big boys' word/spreadsheet suites, this is for you. A multiplatform and multilingual office suite and an open-source project. Compatible with all other major office suites, free to download, and use.
6.Phones for the iPad: Skype, Line2, Whistlestop. Whistle brings free continental US calling to iPad over WiFi or 3G. Whistle works out of the box with the 4th Gen iPod Touch. Whistle provides free inbound calling to your Whistle number, notifying you of calls through Push notifications.
7.Simplenote: Simplenotehelps me keep notes, lists, ideas and more. The notes automatically synchronize with a computer and devices. It's extremely easy to use. Free. http://itunes.apple.com/us/app/simplenote/id289429962?mt=8
8.Firefox: I'm thankful that Windows Explorer is not a part of my work or personal websurfing life. Free. If you don't know how to find and use this, you'd better.
9.Launchy: Launchy is a free open source application launcher for Windows and (as of version 2.1.1) Linux. It indexes shortcuts in the start menu, and files in specific folders w/o opening the start menu. http://www.launchy.net/
10.Goodreader: A superb PDF reader, it was one of the first file management apps for the iPad, and connects to a huge range of different servers and devices. From their site: Quick summary: super-robust PDF reader with advanced reading, annotating, markup and highlighting capabilities, excellent file manager, TXT file reader and editor, audio/video player, Safari-like viewer for MS Office and iWorks files. http://www.goodiware.com/goodreader.html
Wednesday, November 24, 2010
Bayer Settles Multivitamin Prostate Cancer Claim Litigation
Bayer has agreed to a $3.3 million settlement with 3 states "over the company's unsupported claim that One A Day Men's Health Formula multivitamins reduce the risk of prostate cancer," according to the Oregon Department of Justice, which filed a complaint along with the attorney generals of California and Illinois.
Bayer will be barred from claiming that its One A Day multivitamins might cure, treat, or prevent any disease, including cancer, unless the company "can back up such claims with competent and reliable scientific evidence," according to the CSPI.
The heart of the current controversy is related to qualified health claims about selenium, an ingredient in the One A Day Men's Health Formula multivitamin.
Read more here. http://www.medscape.com/viewarticle/731707
Bayer will be barred from claiming that its One A Day multivitamins might cure, treat, or prevent any disease, including cancer, unless the company "can back up such claims with competent and reliable scientific evidence," according to the CSPI.
The heart of the current controversy is related to qualified health claims about selenium, an ingredient in the One A Day Men's Health Formula multivitamin.
Read more here. http://www.medscape.com/viewarticle/731707
Saturday, November 20, 2010
November 20, 2010: Darvocet Recall News
 Image via WikipediaSome background after this week's recall:
Image via WikipediaSome background after this week's recall: The first propoxyphene products were approved based on safety only under the
1938 Food Drug & Cosmetic Act (FD&C Act), and they were Darvon (propoxyphene HCl 32 mg and
65 mg) and a Darvon-Compound (aspirin, caffeine combination) that was later withdrawn.
There were published reviews of medications. The authors made similar conclusions: In one, it was claimed that Propoxyphene, as a single-ingredient product, was a weak analgesic. It was claimed that Propoxyphene had no or little contribution to efficacy of reduction of acute pain.
Darvon was initially developed by Eli-Lilly and now marketed by two companies--Xanodyne Pharmaceuticals of Kentucky and Qualitest/Vintage Pharmaceuticals of Alabama--was first approved for use in the United States in 1957. Darvon is commonly combined with a dose of acetaminophen and marketed by Xanodyne under the name Darvocet. Both drugs are classified as narcotic pain relievers. Darvocet is one of the most commonly prescribed drugs in the US for treating patients with mild to moderate pain, with more than 20 million prescriptions written in 2007.
Dextropropoxyphene did carry a black box warning, stating:
Propoxyphene should be used with extreme caution, if at all, in patients who have a history of substance/drug/alcohol abuse, depression with suicidal tendency, or who already take medications that cause drowsiness (e.g., antidepressants, muscle relaxants, pain relievers, sedatives, tranquilizers). Fatalities have occurred in such patients when propoxyphene was misused.
FDA: Watch our for the Fake Viagra
 Image via Wikipedia (This is the real stuff, not the copycat stuff the FDA's warning you about this weekThe U.S. Food and Drug Administration says Vigor-25, a product marketed as a natural dietary supplement to enhance male sexual performance, should not be purchased or used because it contains sildenafil, the active ingredient in the prescription drug Viagra.
Image via Wikipedia (This is the real stuff, not the copycat stuff the FDA's warning you about this weekThe U.S. Food and Drug Administration says Vigor-25, a product marketed as a natural dietary supplement to enhance male sexual performance, should not be purchased or used because it contains sildenafil, the active ingredient in the prescription drug Viagra.The FDA has found many products marketed as dietary supplements for sexual enhancement during the past several years that can be harmful because they contain active ingredients in FDA-approved drugs or variations of these ingredients. Sexual enhancement products promising rapid effects (e.g., claim to work in minutes to hours) or long-lasting effects (e.g., claim to last 24-72 hours) are likely to contain a contaminant.
The FDA advises consumers who have experienced any negative side effects from sexual enhancement
products to stop using such products and consult a health care professional and to safely discard the product. The FDA urges health care professionals and consumers to report adverse events or side effects from use of Vigor-25 to the FDA's MedWatch Adverse Event Reporting program.
http://www.prnewswire.com/news-releases/fda-warns-consumers-not-to-use-vigor-25-109254364.html
November 20, 2010: Darvon/Darvocet Recall Information and History
 Image by amayzun via FlickrThe news is out, but what do people know about how the recall came to be?
Image by amayzun via FlickrThe news is out, but what do people know about how the recall came to be?The consumer interest group Public Citizen filed a federal lawsuit last year against the U.S. Food and Drug Administration (FDA) claiming that the agency violated the law by failing to act on a petition filed in 2006, which urged the removal of Darvocet, Darvon and generic propoxyphene drugs from the market because they have been associated with over 2,000 accidental deaths, are physically addictive and are no more effective than other safer painkillers.
Darvon and Darvocet contain the narcotic propoxyphene, which is also available as a generic. It has been more than three years since British health authorities cited hundreds of Darvon and Darvocet deaths as the reason they ordered the drug to be phased off of the market, and it has been more than two years since Public Citizen filed their petition urging the FDA to phase the drug out. However, the U.S. drug regulators have not yet taken any actions to protect consumers from the dangerous side effects, and it remains one of the most widely prescribed generic drugs, with approximately 22 million prescriptions in 2007.
The narcotic drug is addictive, yet it is a relatively weak painkiller, say opponents. Public Citizen indicates that it is no more effective at treating most kinds of pain than other safer alternatives, such as ibuprofen (the active ingredient in Advil) and acetaminophen (the active ingredient in Tylenol).
“A large portion of the deaths from propoxyphene occurred because the drug is converted into a metabolite that is highly toxic to the heart and lasts longer in the body than the original compound, resulting in cardiac depression,” said Public Citizen in a statement released February 28, 2006 when they originally filed their petition. “Adverse cardiac events associated with propoxyphene include an interruption of heart transmission of electrical impulses, slowed heartbeats and a decreased ability of the heart to contract properly.”
According to data from the Federal Drug Abuse Warning Network, deaths related to Darvocet, Darvon and other generic propoxyphene drugs account for over 5% of all drug-related deaths between 1987 and 200
November Avandia News
Senator Max Baucus, Chairman of the Committee on Finance, and Senator Chuck Grassley, Ranking Member, released a committee report based on a two year inquiry of the diabetes drug Avandia. The senators also asked the Food and Drug Administration to describe what steps the agency has taken to protect patients in an ongoing Avandia clinical trial, and why the study is allowed to continue, given that the FDA itself estimated that the drug caused approximately 83,000 excess heart attacks between 1999 and 2007.
The committee report quotes the senators as saying, “As senior members of the United States Senate and Chairman and Ranking Member of the Committee on Finance (Committee), we have a duty under the Constitution to conduct oversight into the actions of executive branch agencies, including the Food and Drug Administration (FDA). In this capacity, we must ensure that FDA properly fulfill their mission to advance the public’s welfare, safeguard the nation’s drug supply, and protect patients participating in clinical trials.”
Will the FDA seek a withdrawal of Avandia? Stay tuned
The committee report quotes the senators as saying, “As senior members of the United States Senate and Chairman and Ranking Member of the Committee on Finance (Committee), we have a duty under the Constitution to conduct oversight into the actions of executive branch agencies, including the Food and Drug Administration (FDA). In this capacity, we must ensure that FDA properly fulfill their mission to advance the public’s welfare, safeguard the nation’s drug supply, and protect patients participating in clinical trials.”
Will the FDA seek a withdrawal of Avandia? Stay tuned
Friday, November 19, 2010
Darvon and Darvocet/Propoxyphene News November 19, 2010
The FDA has asked that propoxyphene, (brand names Darvon and Darvocet, Xanodyne Pharmaceuticals) be removed from the US market. The decision will also affect generic manufacturers and the makers of propoxyphene-containing products.
Call us anytime, including after 5 PM and on weekends
The FDA has told medical professionals to stop prescribing propoxyphene. Patients who are taking the stated medications should not sinply stop taking it, ut should contact their doctor soon as possible to discuss switching to another pain-management therapy.
Propoxyphene is an opioid typically used to treat mild to moderate pain. It was first approved by the FDA in 1957. It is sold by prescription under various names alone or in combination with acetaminophen. Since 1978, the FDA has received two requests to remove propoxyphene from the market.
In January 2009, an FDA advisory committee voted 14 to 12 against the continued marketing of propoxyphene products. At that time, the committee called for additional information about the drug's cardiac effects.
Talk to us if you believe that you or a loved one was injured because of this medication. The call is confidential and free.
Reusable Shopping Bags and Possible Lead Contamination?
 Image via Wikipedia The recent report by a newspaper in Tampa, Fl. has caused retailers and the Food and Drug Administration (FDA) to investigate the lead levels of reusable shopping bags.
Image via Wikipedia The recent report by a newspaper in Tampa, Fl. has caused retailers and the Food and Drug Administration (FDA) to investigate the lead levels of reusable shopping bags.Tests showed some Publix reusable bags had lead levels that exceed federal limits for paint and exceeded rules coming soon for children's toys. Though the bags comply with other limits, Publix, in a cautionary move, asked its bag suppliers to lower lead content in bags. That decision came after officials were told the results of tests.
Read more here at the source: http://www2.tbo.com/content/2010/nov/14/140842/lead-taints-reusable-bags/news-metro/
Wednesday, November 17, 2010
Iqbal/Twombly Applies to All Civil Cases in Federal Court, Including State Law Claims Removed to Federal Court
 Image via Wikipedia
Image via WikipediaSo says the TN USDCT, in an opinion released last week.
http://scholar.google.com/scholar_case?case=14595711359472781380&hl=en&as_sdt=2&as_vis=1&oi=scholarr
SALLIE MANESS, Plaintiff,
v.
BOSTON SCIENTIFIC, et al., Defendants.
MEMORANDUM AND ORDER
THOMAS W. PHILLIPS, District Judge.This matter is before the Court on Defendants' Motion to Dismiss [Doc. 8]. On June 4, 2010, Defendants filed a Motion to Dismiss pursuant to Rule 12(b)(6) of the Federal Rules of Civil Procedure. Defendants argue that Plaintiff's claims—all based in products liability—do not satisfy the federal pleading requirements, as modified in Bell Atl. Corp. v. Twombly, 550 U.S. 544 (2007), and Ashcroft v. Iqbal, ___ U.S. ___, 129 S.Ct. 1937 (2009).
On October 19, 2010, Plaintiff responded in opposition, arguing that: (1) federal pleading requirements do not apply to state law claims removed to federal court; and (2) in any event, Plaintiff satisfied the federal pleading requirements, as modified by Twombly. [Plaintiff's Response to Defendants' Motion to Dismiss, Doc. 13]. On October 28, 2010, Defendants filed a Reply in support of their Motion to Dismiss. [Doc. 15].
Based upon the following, Defendants' Motion to Dismiss [Doc. 8] is GRANTED, whereby Plaintiff's complaint is DISMISSED. While Plaintiff's complaint is dismissed, Plaintiff has 30 days from entry of this Memorandum and Order to file an amended complaint. If Plaintiff fails to file an amended complaint within this time period, or if the amended complaint fails to satisfy the federal pleading requirements, judgment shall be entered in favor of the Defendants.
I. BACKGROUND
As an initial matter, the Court notes that it has jurisdiction pursuant to 28 U.S.C. §§ 1332, 1441. The following facts are taken mostly from Plaintiff's complaint, and will be assumed as true for purposes of the 12(b)(6) motion. See, e.g., DIRECTV, Inc. v. Treesh, 487 F.3d 471, 476 (6th Cir. 2007) (in ruling upon motions to dismiss under Rule 12(b)(6), a court must "construe the complaint in the light most favorable to the plaintiff, accept its allegations as true, and draw all reasonable inferences in favor of the plaintiff").On March 16, 2010, Plaintiff filed a product liability action against defendants Boston Scientific Corporation[1], Advanced Bionics[2], Scott Stewart, and John Does 1-5. [Plaintiff's Complaint, Doc. 1-1]. The complaint was filed in the Circuit Court for Knox County. [Id.]. On April 23, 2010, Defendants removed the case to federal court on the basis of diversity jurisdiction, 28 U.S.C. §§1331, 1441. [Defendants' Notice of Removal, Doc. 1].
In her complaint, Plaintiff alleges that she suffered injuries[3] after having a medical device implanted. [Plaintiff's Complaint, Doc. 1-1, ¶ IX, at 6]. In June 2007, at Fort Sanders Regional Medical Center in Knoxville, Tennessee, Plaintiff had a spinal cord simulation system device implanted. [Id.]. This device, the "Implantable Pulse Generator Advanced Bionics Precision SCS, Model number IPG SC-1110" (hereafter, the "Device"), is used to treat back pain. [Id.]. On October 6, 2007, a recall was issued for the model that Plaintiff had implanted. [Notice of Recall, Doc. 1-1 at 12-15]. According to Boston Scientific, only 8 patients out of 12,700 reported problems with the Device. [Field Safety Notice, Doc. 1-1 at 17]. On March 20, 2009, after "much pain and intense suffering and massive infection," Plaintiff had the Device removed.[4] [Plaintiff's Complaint, Doc. 1-1, ¶ IX, at 7].
Plaintiff has filed product liability claims against Boston Scientific and Advanced Bionics, the corporations which allegedly "designed, manufactured, assembled, distributed and sold" the Device. [Id. ¶ V, at 4]. In addition, Plaintiff has sued Scott Stewart, a field sales representative for Boston Scientific, who allegedly "maintained, sold, serviced, controlled, installed and removed" the Device. [Id. ¶ VI, at 5]. Plaintiff has also sued "John Does 1-5," several unidentified defendants [Id.].
Plaintiff has sued Defendants under several theories, including negligence, reckless misconduct, malice, fraud and oppression, and strict liability "in manufacturing, designing, assembling distributing maintaining, repairing, servicing, selling and installation"of the Device," and in "failing to include warnings as to its dangerous propensities and handling characteristics." [Id. ¶X.1, at 7]. While Plaintiff has not clearly identified her causes of action, it appears that she has sued Defendants under theories of defective design, defective manufacturing, and "failure to warn," among others.
On June 4, 2010, Defendants moved to dismiss pursuant to Rule 12(b)(6) of the Federal Rules of Civil Procedure. Defendants argue that Plaintiff failed to satisfy the federal pleading requirements, as modified in Twombly, 550 U.S. 544, and Iqbal, 129 S.Ct. 1937. On October 19, 2010, Plaintiff responded in opposition. [Plaintiff's Response to Defendants' Motion to Dismiss, Doc. 13]. On October 28, 2010, Defendants filed a Reply in support of their Motion to Dismiss [Doc. 16]. The matter is now ripe for adjudication.
II. STANDARD OF REVIEW
Under Rule 8(a)(2) of the Federal Rules of Civil Procedure, a complaint must contain "a short and plain statement of the claim showing that the pleader is entitled to relief." Fed. R. Civ. P. 8(a)(2). In 2007, the Supreme Court modified the pleading standard in the context of antitrust cases. Twombly, 550 U.S. at 570. Notably, the Supreme Court held that in order to survive a 12(b)(6) motion to dismiss—which attacks the sufficiency of a complaint—the plaintiff must "state a claim to relief that is plausible on its face." Id. (emphasis added). In 2009, the Supreme Court extended the Twombly (or plausibility) standard to all federal civil cases. Iqbal, 129 S.Ct. at 1953.Under the new standard, a claim is facially plausible if the plaintiff "pleads factual content that allows the court to draw the reasonable inference that the defendant is liable for the misconduct alleged." Id. at 1949 (citing Twombly, 550 U.S. at 556). While this is not akin to a "probability requirement," the plaintiff must show "more than a sheer possibility that a defendant has acted unlawfully." Id. (citing Twombly, 550 U.S. at 556). This "requires more than labels and conclusions, and a formulaic recitation of the elements of a cause of action will not do." Twombly, 550 U.S. at 555 (citations and quotation marks omitted). In other words, a plaintiff must "plead factual content that allows the court to draw the reasonable inference that the defendant is liable for the misconduct alleged." Iqbal, 129 S.Ct. at 1949. A plaintiff falls short if he pleads facts "merely consistent with a defendant's liability" or if the alleged facts do not "permit the court to infer more than the mere possibility of misconduct . . ." Id.
In ruling upon motions to dismiss under Rule 12(b)(6), a court must "construe the complaint in the light most favorable to the plaintiff, accept its allegations as true, and draw all reasonable inferences in favor of the plaintiff." DIRECTV, 487 F.3d at 476. However, the court "need not accept as true legal conclusions or unwarranted factual inferences." Id. (quoting Gregory v. Shelby Cnty., 220 F.3d 433, 446 (6th Cir. 2000)). See also Iqbal, 129 S.Ct. at 1949 ("[T]he tenet that a court must accept as true all of the allegations contained in a complaint is inapplicable to legal conclusions. Threadbare recitals of the elements of a cause of action, supported by mere conclusory statements, do not suffice.") (citing Twombly, 550 U.S. at 555). Ultimately, this determination-whether a plaintiff's claim is "plausible"—is a "context-specific task that requires the reviewing court to draw on its judicial experience and common sense." Iqbal, 129 S.Ct. at 1950 (citations omitted).
III. ANALYSIS
A. Twombly Applies to All Civil Cases in Federal Court, Including State Law Claims Removed to Federal Court
As an initial matter, Plaintiff argues that the Court should look to Tennessee law for pleading requirements. [Plaintiff's Response to Defendants' Motion to Dismiss, Doc. 13 at 4]. In other words, Plaintiff wants the Court to apply Tennessee law—instead of Twombly—in ruling upon the 12(b)(6) motion. [Id.]. According to Plaintiff, the Court "should follow state law pleading requirements since this is a claim based upon state law . . ." [Id.].Plaintiff's argument is without merit. It does not matter whether Plaintiff's claims are based upon state law or federal law: all claims, once removed to federal court, are subject to federal pleading requirements. In Iqbal,. the Supreme Court held that Twombly's plausibility standard applies to all civil cases in federal court. 129 S.Ct. at 1953. In Iqbal, the respondents argued that the plausibility standard should be limited to antitrust cases, as in Twombly. Id. The Supreme Court rejected this argument, holding that the plausibility standard applies to all civil cases in federal court:
Though Twombly determined the sufficiency of a complaint sounding in antitrust, the decision was based on our interpretation and application of Rule 8. That Rule in turn governs the pleading standard in all civil actions and proceedings in the United States district courts.Id. (emphasis added) (citations and quotations omitted). See also Minger v. Green, 239 F.3d 793, 799-801 (6th Cir. 2001) (applying federal pleading rules in a case based upon diversity jurisdiction); Wilkey v. Hull, 366 F. App'x 634, 637 (6th Cir. 2010) (applying Twombly pleading standard in a diversity case to determine whether the plaintiff alleged sufficient facts to support state law claims); Foust v. Stryker Corp., No. 2:10-cv-00005, 2010 WL 2572179, at *2 (S.D. Ohio Jun. 22, 2010) (applying Twombly pleading standard to a complaint alleging product liability claims under Ohio law).
Although the complaint was filed in state court, it was eventually removed to federal court. As a result, the complaint is subject to federal pleading requirements, which includes Rule 8(a)(2) of the Federal Rules of Civil Procedure. See Granny Goose Foods, Inc. v. Brotherhood of Teamsters & Auto Truck Drivers Local No. 70 of Alameda Cnty., 415 U.S. 423, 438 (1974) ("The Federal Rules of Civil Procedure, like other provisions of federal law, govern the mode of proceedings in federal court after removal.") (emphasis added); Fed R. Civ. P. 81(c) ("These rules apply to civil actions removed to the United States district courts from the state courts and govern procedure after removal.") (emphasis added). In Twombly, 550 U.S. at 550, and Iqbal, 129 S.Ct. at 1953, the Supreme Court modified Rule 8(a)(2) by incorporating a "plausibility" standard. Accordingly, the Court must determine whether Plaintiff has "state[d] a claim to relief that is plausible on its face." Twombly, 550 U.S. at 570 (emphasis added).
B. Plaintiff's Complaint Does Not Satisfy Twombly's Pleading Requirements
1. Introduction
Defendants argue that Plaintiff's complaint does not satisfy Twombly because it does not "plead sufficient factual allegations concerning how the product was purportedly defective, and how the purported defect caused her alleged injury." [Defendants' Reply in Support of their Motion to Dismiss, Doc. 15 at 3]. In response, Plaintiff argues that her complaint satisfies Twombly because it put Defendants "on notice that this it [sic] is a products liability case, that the Defendants[s] are the maker of a defective product, and that this defective product had to be removed from the Plaintiff's body." [Plaintiff's Response in Opposition to Defendants' Motion to Dismiss, Doc. 14 at 3]. Having reviewed Plaintiff's complaint, the Court finds that it fails to allege sufficient facts to support a product liability action.While Plaintiff has filed several claims against Defendants, each is covered by the Tennessee Product Liability Act of 1978 ("TPLA"), T.C.A. §§ 29-28-101 et seq.[5] See Higgs v. Gen. Motors Corp., 655 F. Supp. 22, 23 (E.D. Tenn. 1985) ("Indeed, it makes no difference whether the complaint is couched in terms of negligence, strict liability or breach of warranty, it has generally been held in the State of Tennessee that in order for a plaintiff to recover under any theory of product liability, the plaintiff must establish that the product was defective and unreasonably dangerous at the time the product left the control of the manufacturer."). Regardless of Plaintiff's theory of recovery—which includes strict liability, defective design, defective manufacturing, failure to warn—Plaintiff must allege facts for the Court to infer that the Device was "defective" or "unreasonably dangerous" at the time it left the control of the manufacturer. See King v. Danek Med., Inc., 37 S.W.3d 429, 435 (Tenn. Ct. App. 2000) ("Unless the product was in a defective condition or unreasonably dangerous when it left the control of [the manufacturer], there is no liability pursuant to [the TPLA]"); T.C.A § 29-28-105(a) ("A manufacturer or seller of a product shall not be liable for any injury to person or property caused by the product unless the product is determined to be in a defective condition or unreasonably dangerous at the time it left the control of the manufacturer or seller.") (emphasis added). At this stage in the proceeding, Plaintiff must allege facts for the Court to infer that: "(1) the product was defective and/or unreasonably dangerous, (2) the defect existed at the time the product left the manufacturer's control, and (3) the plaintiff's injury was proximately caused by the defective product." Sigler v. Am. Honda Motor Co., 532 F.3d 469, 483 (6th Cir. 2008) (citing King, 37 S.W.3d at 435). Under the TPLA, a product is "defective" if it is a "product that renders it unsafe for normal or anticipate handling and consumption." T.C.A. § 29-28-102(2). In addition, a product is "unreasonably dangerous" if it is "dangerous to an extent beyond that which would be contemplated by the ordinary consumer who purchases it, with the ordinary knowledge common to the community as to its characteristics, or that the product because of its dangerous condition would not be put on the market by a reasonably prudent manufacturer or seller assuming that he knew of its dangerous condition." T.C.A. § 29-28-102(8).
In Tennessee, there are two tests for determining whether a product is unreasonably dangerous. See T.C.A. § 29-28-102(8). Under the "consumer expectation test," this "requires a showing that the product's performance was below reasonable minimum safety expectations of the ordinary consumer having ordinary, `common' knowledge as to its characteristics." Jackson v. Gen. Motors Corp., 60 S.W.3d 800, 806 (Tenn. 2001). Under the "prudent-manufacturer test," the Court "imputes knowledge of the dangerous condition to the manufacturer, and then asks whether, given that knowledge, a prudent manufacturer would market the product. As the Tennessee Supreme Court has articulated, `[t]he consumer expectation test is, by definition, buyer oriented; the prudent manufacturer test, seller oriented.'" Johnson v. Manitowoc Boom Trucks, Inc., 484 F.3d 426, 428-29 (6th Cir. 2007) (citations omitted). Only sellers and manufacturers may be held liable under the TPLA.[6] See T.C.A. § 29-28-102(4), (7).
In all product liability actions, "[a] plaintiff must show that there was something wrong with the product, and trace the plaintiff's injury to the specific defect." King, 37 S.W.3d at 435 (citations omitted). See also Browder v. Pettigrew, 541 S.W.2d 402, 404 (Tenn. 1976) (holding that in order to establish a defective design claim, the plaintiff must "trace the injury to some specific error in construction or design of the [product]" and that "in a products liability action in which recovery is sought under the theory of negligence, the plaintiff must establish the existence of a defect in the product just as he does in an action where recovery is sought under the strict liability theory or for breach of warranty, either express or implied.") (citations omitted). At this stage in the proceedings, Plaintiff must allege facts for the Court to infer that the Device was defective, and that Plaintiff's injuries were caused by the condition of the Device. In this case, Plaintiff has failed to do both.
2. Plaintiff Failed to Allege Sufficient Facts for the Court to Infer that the Device Was Defective or Unreasonably Dangerous
The fact that Plaintiff allegedly suffered an injury from the Device does not show that the Device was defective. See King, 37 S.W.3d at 435 ("[i]n a product liability claim, the fact that a plaintiff is injured is not proof of a defect in the product") (citing Whaley v. Rheem Mfg. Co., 900 S.W.2d 296, 299 (Tenn. Ct. App. 1995); King, 37 S.W. 3d at 435 (recognizing that "the failure or malfunction of the device, without more, will not make the defendant liable") (citing Harwell v. Am. Med. Sys., Inc., 803 F. Supp. 1287, 1298 (M.D. Tenn. 1992)); Allen v. Am. Med. Sys., Inc., 1989 WL 105626 (Tenn. Ct. App. Sept. 15, 1989) (declining to find liability based upon the fact that the device did not function). Even assuming that the Device injured Plaintiff, that is not enough to show that the Device was defective or unreasonably dangerous—an element necessary for each of Plaintiff's claims. Instead of alleging facts for the Court to infer that the Device was defective or unreasonably dangerous, Plaintiff asserts conclusory allegations. For example, Plaintiff alleges that the "defective medical device [the Device] was not fit for the purpose intended and was defective and therefore caused the plaintiff harm." [Plaintiff's Complaint, Doc. 1-1, ¶ IV, at 6].The facts of the present case are similar to Frey v. Novartis Pharm. Corp, 642 F. Supp. 2d 787 (S.D. Ohio 2009). In Frey, the plaintiff filed a product liability action in state court against the manufacturer of an anti-seizure medication. Id. at 790. The plaintiff alleged that she suffered health complications after ingesting the medication. Id. The plaintiff sued the manufacturer under theories of defective design and manufacturing (under Ohio law). Id. In support of her claims, the plaintiff alleged the following:
Defendants failed to design, manufacture, test, and control the quality of [the anti-seizure medication] such that when it left the control of the Defendant, it deviated in a material way from the design specification, formula or performance standards of the manufacturer, or from otherwise identical units manufactured to the same design specifications, formula or performance standards.
As a direct and proximate result of the defect in manufacture or construction by Defendants, Plaintiff [] suffered the injuries [] and damages set forth herein.Id.
The defendant manufacturer removed the action to federal court, and then moved to dismiss under Rule 12(b)(6). Id. In particular, the defendant argued that the complaint failed to satisfy Twombly's pleading requirements. In response, the plaintiff argued that she could not "particularly allege that the scientific makeup of the drug is defective for a specific reason without conducting discovery, which requirement would exceed Twombly's plausibility standard." Id. at 792. The district court dismissed the defective design and manufacturing claims, finding that the complaint failed to satisfy Twombly:
Plaintiffs' first cause of action for strict liability for defect in the manufacture [and design] of [the anti-seizure medication] . . . must be dismissed pursuant to Rule 12(b)(6) for failure to state a plausible claim for relief. Plaintiffs have done nothing more than provide a formulaic recitation of the elements of a claim under the statute. They have failed to allege any facts that would permit the Court to conclude that a manufacturing defect [or design defect] occurred and that the defect was the proximate cause of [the Plaintiffs'] alleged injuries. Plaintiffs' allegations in this regard fall far short of the sufficiency standard set forth in Twombly.Id. at 795.
Like the plaintiff in Frey, Plaintiff in the present case asserts legal conclusions about the nature of the Device. For example, Plaintiff alleges that Defendants "failed to warn" others of the "dangerous propensities and handling characteristics" of the Device. [Plaintiff's Complaint, Doc. 1-1, ¶ X.1, at 7]. However, Plaintiff does not allege any facts for the Court to infer that the Device was unreasonably dangerous. To plead a "failure to warn" claim[7], Plaintiff must allege facts for the Court to infer that the Device was "unreasonably dangerous" within the meaning of T.C.A. § 29-28-102(8). See Evridge v. Am. Honda Motor Co., 685 S.W.2d 632, 636 (Tenn. 1985) (defining an adequate warning-which is a defense to a "failure to warn" claim—as "one calculated to bring home to a reasonably prudent user of the product the nature and the extent of the danger involved in using the product") (emphasis added). Regardless of the theory of recovery—whether it be breach of warranty, negligence, defective design-Plaintiff must allege facts for the Court to infer that the Device was defective or unreasonably dangerous. See, e.g., Friedman v. Intervet Inc., No. 3:09CV2945, 2010 WL 2817257, at *3, 4 (S.D. Ohio Jul. 16, 2010) (denying a defendant manufacturer's motion to dismiss a product liability action because "[u]nlike Frey, in this case, plaintiff alleges specific problems with the product [a veterinary pharmaceutical used to treat diabetes in animals]; namely, that test results showed the product was out of specification with regard to its primary compound, and that this was a deviation from the product's intended characteristics" and that "[p]laintiff's allegations-detailing the product's problem . . . are more than sufficient to nudge [] [his] claims across the line from conceivable to plausible") (emphasis added) (quotations and citation omitted). Like the plaintiff in Frey, Plaintiff failed to satisfy Twombly's plausibility standard because she did not allege facts for the Court to infer that the Device was defective or unreasonably dangerous.
3. Plaintiff Failed to Allege Sufficient Facts for the Court to Infer a Causal Connection Between the Device's Condition and Her Alleged Injuries
Plaintiff's complaint fails for a second reason: she did not allege facts for the Court to infer that the condition of the Device—based upon an alleged design or manufacturing defect—caused her alleged injuries. As Tennessee courts make clear, it is not enough that Plaintiff suffered injuries from using (or in this case, having implanted) a product. See, e.g., Browder, 541 S.W.2d at 404 (holding that in order to establish a product liability claim, the plaintiff must "trace the injury to some specific error in construction or design of the [product]"). While Plaintiff alleges that the Device caused her pain, she does not allege facts regarding how an alleged defect—whether it be in design or manufacturing—caused her injuries. The relevant question is not whether the Device caused her pain; the issue is whether the alleged defective design or manufacturing of the Device caused her pain. See id. Plaintiff has not alleged any facts regarding this issue.On a final note, the Court finds Plaintiff's allegations regarding a recall insufficient to support her product liability claims. While a Notice of Recall [Doc. 1-1, at 12-15] was issued in February 2008[8], Plaintiff alleges that she was not informed until much later. [Plaintiff's Complaint, Doc. 1-1, ¶ X.2, at 8]. In particular, Plaintiff alleges that "the defective device remained in the plaintiffs' [sic] person causing pain and injury for a period of more than sixteen months after the recall of this defective device, which was unknown and never at anytime published to the plaintiff. Plaintiff believes and avers that the facts and circumstances which lead to the recall of this defective device and that they were known to the defendants at the time, or shortly thereafter, its installment into the plaintiffs' body." [Id.]. In support of her product liability claims, Plaintiff attached the Notice of Recall to her complaint. [Doc. 1-1, at 12-15]
In ruling upon a Rule 12(b)(6) motion to dismiss, courts consider the complaint as well as "documents incorporated into the complaint by reference, and matters of which a court may take judicial notice." Bowers v. Wynne, 615 F.3d 455, 470 (6th Cir. 2010) (emphasis added) (quoting Tellabs, Inc. v. Makor Issues & Rights, Ltd., 551 U.S. 308, 322 (2007)). See also Bassett v. NCAA, 528 F.3d 426, 430 (6th Cir. 2008) ("When a court is presented with a Rule 12(b)(6) motion, it may consider the Complaint and any exhibits attached thereto . . . so long as they are referred to in the Complaint and are central to the claims contained therein.") (citation omitted). Because the complaint expressly mentions the recall, and because it is central to Plaintiff's product liability claims, the Notice of Recall [Doc. 1-1 at 12-15] will be incorporated by reference. The Notice of Recall, which was issued on February 27, 2008, states that the Device was recalled for the following reason:
Incorrect Data-Corruption of internal memory component results in an inability for the physician to reprogram the IPG [Implantable Pulse Generator, the type of device that Plaintiff had implanted] with firmware version prior to Revision 3.02. When this occurs, the IPG will report an error code of `10h0' or `00h0' through the Remote Control. Under this condition, the IPG will cease to log in some data that could be used for informational purposes. . . .[Id. at 14]. In addition, Boston Scientific issued a Field Safety Notice[9] on October 24, 2007, stating the following:
This condition does not affect routine IPG functionality. The IPG delivers the same therapy as it would without the error, and the Remote Control continues to allow the full range of simulation parameter adjustment. The IPG, however, will cease logging of some data that could be used for informational purposes.[Field Safety Notice, Doc. 1-1 at 16]. The Field Safety Notice states that only "8 patients out of over 12,7000 implanted IPGs (0.063%)" suffered from the recall problem. [Id. at 17].
While Plaintiff attached the Notice of Recall [Doc. 1-1 at 12-15] and Field Safety Notice [Doc. 1-1 at 16-17] to her complaint, she failed to allege facts for the Court to infer a connection between the recall, the condition of the Device, and her alleged injuries. Notably, Plaintiff does not allege that the Device malfunctioned, or that the Device suffered from the recall problem. Moreover, it does not appear that the recall was even motivated by a safety concern. As Defendants explain, the recall "was related to a software issue in the device's remote control memory, which would result in an error code being displayed on the remote control screen." [Defendants' Memorandum of Law in Support of their Motion to Dismiss, Doc. 9 at 3]. In sum, Plaintiff failed to allege facts regarding: (1) whether the recall was based upon injuries to other persons, or a concern about future injuries; (2) whether the Device suffered from the recall problem; and (3) assuming that the Device suffered from the recall problem, whether such condition caused her alleged injuries.
IV. CONCLUSION
As the Supreme Court stated in Iqbal, "[a] pleading that offers `labels and conclusions' or `a formulaic recitation of the elements of a cause of action will not do.'" Iqbal, 129 S.Ct. at 1949 (quoting Twombly, 550 U.S. at 555). Plaintiff's complaint fails for two reasons. First, Plaintiff failed to allege facts for the Court to infer that the Device was defective or unreasonably dangerous. Second, Plaintiff failed to allege facts for the Court to infer that the product's condition caused Plaintiff's alleged injuries.Based upon the foregoing, Defendants' Motion to Dismiss [Doc 8] is GRANTED, whereby Plaintiff's complaint is DISMISSED. However, Plaintiff is granted 30 days from entry of this Memorandum and Order to file an amended complaint. If Plaintiff fails to file an amended complaint within this time period, or if the amended complaint fails to satisfy federal pleading requirements, judgment shall be entered in favor of the Defendants.
IT IS SO ORDERED.
Subscribe to:
Posts (Atom)















