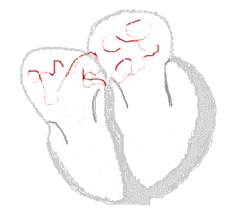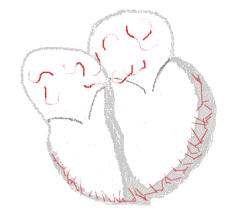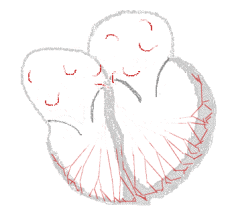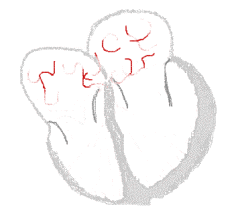 Image via Wikipedia
Image via WikipediaFinal results from the PALLAS study showed that risk of stroke, heart attack or death from cardiovascular causes was 2.2 times greater for patients taking Multaq (dronedarone) than for those on placebo.
The illustration to the right shows atrial fibrillation. A-fib) is the most common cardiac arrhythmia (abnormal heart rhythm). It is a common cause of irregular heart beat, identified clinically by taking a pulse. Chaotic electrical activity in the two upper chambers (atria) of the heart result in the muscle fibrillating (i.e., quivering), instead of achieving coordinated contraction. The presence of AF can be confirmed with an electrocardiogram (ECG or EKG) by the absence of P waves and an irregular ventricular rate. Presence of AF in a population increases with age, with 8% of people over 80 having AF
The illustration to the right shows atrial fibrillation. A-fib) is the most common cardiac arrhythmia (abnormal heart rhythm). It is a common cause of irregular heart beat, identified clinically by taking a pulse. Chaotic electrical activity in the two upper chambers (atria) of the heart result in the muscle fibrillating (i.e., quivering), instead of achieving coordinated contraction. The presence of AF can be confirmed with an electrocardiogram (ECG or EKG) by the absence of P waves and an irregular ventricular rate. Presence of AF in a population increases with age, with 8% of people over 80 having AF
Multaq is licensed to treat adults who have had atrial fibrillation (AF) in the past, or who currently have intermittent AF, but PALLAS was evaluating it in patients who were 65 years old or more with permanent AF and other risk factors for vascular events.
The trial was halted in July after researchers found evidence of serious vascular events and deaths - less than a third of the planned 10,800 patients eventually took part.
Delegates at the American Heart Association’s Scientific Sessions 2011 were told that 43 people taking Multaq, and 19 in the placebo arm, suffered a stroke, heart attack, systemic blood clot or cardiovascular death.
