Pfizer announced it is voluntarily withdrawing Lipitor advertising and promotion featuring Dr. Robert Jarvik.
For more, go here.
News, musings and commentary on dietary supplements & pharmaceutical law issues, technology, and litigation. Lawyers for consumers and injured people.(No advice on this blog, though) mark(at)markzamora.com
Tuesday, February 26, 2008
Friday, February 22, 2008
Vioxx Update for February, 2008
Lawyers will have an extra month to send in paperwork for people who want early payment from a $4.85 billion federal court settlement involving the painkiller Vioxx.
February 29th is the deadline for "enrolling" people to get the early payments from Merck & Co., which sold the drug until September 2004. Several lawyers are having difficulty gathering the required paperwork, so the deadline for that has been moved to March 30, 2008.
Those who have submitted their names and Social Security numbers by Feb. 29 and their medical and other supporting paperwork by March 30 could get partial payments by late summer. As of now, the 'census' of registrants is 58,000 people as interested in the settlement; more than 13,200 are formally enrolled.
According to various reports and to lawyers I am working with, there may be as many as 27,000 potential enrollees who claim that Vioxx cased a heart attack or stroke.
At the hearing yesterday, my friend Woody Wilner asked Judge Fallon to allow approximately 230 of his clients to be added to the list of those eligible for the settlement. According to Wilner, there were agreements with the committee of lawyers set up to represent plaintiffs in the federal litigation before Fallon, and the agreements had been in place before the settlement had been announced. The 230 had not had their individual cases filed before November 9, 2007 because the Florida SOL had not yet run and Wilner and other attorneys had no way to know the settlement's limits because Fallon had ordered secret negotiations.
Merck and the PSC committee disagreed with Wilner's argument. Stay tuned.
February 29th is the deadline for "enrolling" people to get the early payments from Merck & Co., which sold the drug until September 2004. Several lawyers are having difficulty gathering the required paperwork, so the deadline for that has been moved to March 30, 2008.
Those who have submitted their names and Social Security numbers by Feb. 29 and their medical and other supporting paperwork by March 30 could get partial payments by late summer. As of now, the 'census' of registrants is 58,000 people as interested in the settlement; more than 13,200 are formally enrolled.
According to various reports and to lawyers I am working with, there may be as many as 27,000 potential enrollees who claim that Vioxx cased a heart attack or stroke.
At the hearing yesterday, my friend Woody Wilner asked Judge Fallon to allow approximately 230 of his clients to be added to the list of those eligible for the settlement. According to Wilner, there were agreements with the committee of lawyers set up to represent plaintiffs in the federal litigation before Fallon, and the agreements had been in place before the settlement had been announced. The 230 had not had their individual cases filed before November 9, 2007 because the Florida SOL had not yet run and Wilner and other attorneys had no way to know the settlement's limits because Fallon had ordered secret negotiations.
Merck and the PSC committee disagreed with Wilner's argument. Stay tuned.
Thursday, February 21, 2008
What A Doctor Employed by the FDA said about the FDA
Continuing on the Riegel set of posts, it seems that those applauding the decision conclude that if the FDA can just get funded properly, we'll be fine.
I wanted to see what someone within the FDA thinks of the FDA, and I found:
Dr. David Graham, Associate Director for Science and Medicine in FDA's Office of Drug Safety. He provided testimony to a Senate Committee on the FDA's failures. Compelling stuff.
On one website, you can read this, among other things (and remember this is from someone within the FDA): "I would argue that the FDA, as currently configured, is incapable of protecting America against another Vioxx. We are virtually defenseless."
More:
It is important that this Committee and the American people understand that what has happened with Vioxx is really a symptom of something far more dangerous to the safety of the American people. Simply put, FDA and its Center for Drug Evaluation and Research are broken.
In 1938, Congress enacted the Food, Drug and Cosmetic Act, basically creating the FDA, in response to an unfortunate incident in which about 100 children were killed by elixir of sulfanilamide, a medication that was formulated using anti-freeze. This Act required that animal toxicity testing be performed and safety information be submitted to FDA prior to approval of a drug.
In 1962, Congress enacted the Kefauver-Harris Amendments to the FD&C Act, in response to the thalidomide disaster in Europe.
In my opinion, the FDA has let the American people down (addressing the Vioxx debacle), and sadly, betrayed a public trust. I believe there are at least 3 broad categories of systemic problems that contributed to the Vioxx catastrophe and to a long line of other drug safety failures in the past 10 years.
Read more here.
I wanted to see what someone within the FDA thinks of the FDA, and I found:
Dr. David Graham, Associate Director for Science and Medicine in FDA's Office of Drug Safety. He provided testimony to a Senate Committee on the FDA's failures. Compelling stuff.
On one website, you can read this, among other things (and remember this is from someone within the FDA): "I would argue that the FDA, as currently configured, is incapable of protecting America against another Vioxx. We are virtually defenseless."
More:
It is important that this Committee and the American people understand that what has happened with Vioxx is really a symptom of something far more dangerous to the safety of the American people. Simply put, FDA and its Center for Drug Evaluation and Research are broken.
In 1938, Congress enacted the Food, Drug and Cosmetic Act, basically creating the FDA, in response to an unfortunate incident in which about 100 children were killed by elixir of sulfanilamide, a medication that was formulated using anti-freeze. This Act required that animal toxicity testing be performed and safety information be submitted to FDA prior to approval of a drug.
In 1962, Congress enacted the Kefauver-Harris Amendments to the FD&C Act, in response to the thalidomide disaster in Europe.
In my opinion, the FDA has let the American people down (addressing the Vioxx debacle), and sadly, betrayed a public trust. I believe there are at least 3 broad categories of systemic problems that contributed to the Vioxx catastrophe and to a long line of other drug safety failures in the past 10 years.
Read more here.
A Riegel View From a Nonlawyer?
Some serious gloating (and teeth gnashing) going on all over the net today as a result of Reigel. At a blog discussing drugs and devices, there is commentary on blog posts today.
You can find a good roundup of posts on the New York Personal Injury Law Blog.
Back to the device blog -- taking one set of comments at face value, this poster (with a reference to the Pharmalot blog) writes:
"Everything we have learned about industry, FDA, and their relationship over the past decade (and much longer) tells us that they will not step up to the plate. On the FDA side, they simply do not have the means to do the kind of job you describe.
I believe it a certainty, therefore, that disaster will occur, and it will be on a scale much larger and more devastating than anything we have seen because most of it will happen in the dark. When the levee breaks, it will entail more than burying the bodies and hoping for a Congressional fix. Trust in the FDA and the industry will be shattered for a very, very long time. And that itself will result in both economic and public health disaster. We will have a lot of dead people. And a lot of dead companies. So I agree - the situation will be profoundly worse for the industry, and all of us, than it is now.
That is one reason I have been arguing for several years that preemption has never been in industry’s interest. Entirely, the opposite. And the disapproval rates one sees now will be looked back upon as very good days compared to what is to come.
I am sorry to be so negative. Trust me, this is not a political statement. And it is precisely the opposite of anti-industry. It is looking a policy in the face - the policy of preemption - and simply saying what cannot be blinked away: it is logically, practically, and ethically bankrupt. It is a preemptive strike that will cost more, in both lives and treasure, than the preemptive strike on Iraq. It is an avoidable disaster (in the drug arena), but one that will almost certainly not be avoided.
And, sad to say, those who are on the inside of the industry know this best of all. And many of them have told me this, just as I am sure, in one way or another, they have told you. "
Source here.
I'd like to welcome back to reality anyone who rationally thinks that the FDA is doing a good job now.
You can find a good roundup of posts on the New York Personal Injury Law Blog.
Back to the device blog -- taking one set of comments at face value, this poster (with a reference to the Pharmalot blog) writes:
"Everything we have learned about industry, FDA, and their relationship over the past decade (and much longer) tells us that they will not step up to the plate. On the FDA side, they simply do not have the means to do the kind of job you describe.
I believe it a certainty, therefore, that disaster will occur, and it will be on a scale much larger and more devastating than anything we have seen because most of it will happen in the dark. When the levee breaks, it will entail more than burying the bodies and hoping for a Congressional fix. Trust in the FDA and the industry will be shattered for a very, very long time. And that itself will result in both economic and public health disaster. We will have a lot of dead people. And a lot of dead companies. So I agree - the situation will be profoundly worse for the industry, and all of us, than it is now.
That is one reason I have been arguing for several years that preemption has never been in industry’s interest. Entirely, the opposite. And the disapproval rates one sees now will be looked back upon as very good days compared to what is to come.
I am sorry to be so negative. Trust me, this is not a political statement. And it is precisely the opposite of anti-industry. It is looking a policy in the face - the policy of preemption - and simply saying what cannot be blinked away: it is logically, practically, and ethically bankrupt. It is a preemptive strike that will cost more, in both lives and treasure, than the preemptive strike on Iraq. It is an avoidable disaster (in the drug arena), but one that will almost certainly not be avoided.
And, sad to say, those who are on the inside of the industry know this best of all. And many of them have told me this, just as I am sure, in one way or another, they have told you. "
Source here.
I'd like to welcome back to reality anyone who rationally thinks that the FDA is doing a good job now.
SCOTUS Riegel Opinion: Preemption as to Medical Devices
The U.S. Supreme Court issued its opinion in Reigel. You may find it here.
The ruling will affect the medical devices most frequently targeted in lawsuits, cutting-edge products such as bone screws and defibrillator wires that undergo the FDA'S pre-market approval process. Because that process typically takes almost a year, companies use it relatively sparingly, filing only 43 new applications with the FDA in 2005.
From one blog:
"The medical device industry, or at least the most innovative part of it, received major relief from product liability litigation yesterday in Riegel v. Medtronic (now online at 2008 WL 440744)." As the blogger posted: "As long as [device makers] with PMA-approved devices comply with federal law, [device makers are] not going to be subject to much in the way of product liability." Source here.
For more news, go here.
The ruling will affect the medical devices most frequently targeted in lawsuits, cutting-edge products such as bone screws and defibrillator wires that undergo the FDA'S pre-market approval process. Because that process typically takes almost a year, companies use it relatively sparingly, filing only 43 new applications with the FDA in 2005.
From one blog:
"The medical device industry, or at least the most innovative part of it, received major relief from product liability litigation yesterday in Riegel v. Medtronic (now online at 2008 WL 440744)." As the blogger posted: "As long as [device makers] with PMA-approved devices comply with federal law, [device makers are] not going to be subject to much in the way of product liability." Source here.
For more news, go here.
Wednesday, February 20, 2008
Wyeth Wins a Thimerosal Case
There are claims that Thimerosal is linked to Autism. Jude Stuart R. Berger of the Circuit Court for Baltimore City in Baltimore, Maryland, granted in a Thimerosal case Wyeth's motion for summary judgment. The case was Blackwell v. Sigma Aldrich, Inc., and the claim related to the drug. The Complaint alleged that Jamarr Blackwell's exposure to thimerosal-containing vaccines caused him to become autistic.
For more, go here.
For more, go here.
Tuesday, February 19, 2008
Jury Verdict for My Friend Angel Reyes and His Firm
My friends at Heygood, Orr, Reyes, Pearson & Bartolomei pass along news of a great jury verdict for their client, Tony Alardin, in a partnership dispute regarding the development, manufacture and sale of wireless video surveillance trailer systems.
In 2001, Mr. Alardin, along with his company Remote Monitoring Technologies, entered into a partnership with Dallas businessman Gregg Hoss and his company, Hoss Equipment Company, to perfect the technology and further develop and market wireless video surveillance trailer systems. In March 2005, as the technology was being perfected and as the market became poised for growth, Mr. Hoss barred Mr. Alardin from the premises of the partnership. Hoss Equipment Company continued developing and marketing the wireless video surveillance trailer systems and subsequently generated approximately $2 million in revenue. Mr. Hoss denied the existence of any legal partnership with Mr. Alardin and took the position that his relationship with Mr. Alardin was, at best, a marketing partnership and as a result, he was fully within his rights to do what he did.
In January 2006, Mr. Hoss and Hoss Equipment Co., brought suit against Mr. Alardin seeking, among other things, repayment of the money put into the wireless video surveillance trailer systems prior to the lock-out. Mr. Hoss claimed the money had not been capital contributions to a legal partnership but instead loans to Mr. Alardin and his company that were never repaid.
Mr. Alardin approached Heygood, Orr, Reyes, Pearson & Bartolomei to defend him against the allegations brought against him and to pursue counterclaims against Gregg Hoss, including breach of fiduciary duty. The case was tried in Dallas County before Judge Jim Jordan. After a week and a half trial and two days of deliberation, the jury returned its verdict and found that Mr. Hoss had breached his fiduciary duty to Mr. Alardin. The jury awarded Mr. Alardin $3 million.
Michael Heygood, Partner of HORP&B, was the lead trial attorney for Tony Alardin, and was assisted at trial by Ryan Browne. Mr. Heygood commented, “At the end of the day, as is often the case, the jury was able to understand what was really going on.” According to Mr. Heygood, “Even though there was no written partnership agreement, the jury recognized the partnership between Mr. Hoss and Mr. Alardin and agreed that Mr. Hoss failed to treat Mr. Alardin fairly or justly."
Well done.
In 2001, Mr. Alardin, along with his company Remote Monitoring Technologies, entered into a partnership with Dallas businessman Gregg Hoss and his company, Hoss Equipment Company, to perfect the technology and further develop and market wireless video surveillance trailer systems. In March 2005, as the technology was being perfected and as the market became poised for growth, Mr. Hoss barred Mr. Alardin from the premises of the partnership. Hoss Equipment Company continued developing and marketing the wireless video surveillance trailer systems and subsequently generated approximately $2 million in revenue. Mr. Hoss denied the existence of any legal partnership with Mr. Alardin and took the position that his relationship with Mr. Alardin was, at best, a marketing partnership and as a result, he was fully within his rights to do what he did.
In January 2006, Mr. Hoss and Hoss Equipment Co., brought suit against Mr. Alardin seeking, among other things, repayment of the money put into the wireless video surveillance trailer systems prior to the lock-out. Mr. Hoss claimed the money had not been capital contributions to a legal partnership but instead loans to Mr. Alardin and his company that were never repaid.
Mr. Alardin approached Heygood, Orr, Reyes, Pearson & Bartolomei to defend him against the allegations brought against him and to pursue counterclaims against Gregg Hoss, including breach of fiduciary duty. The case was tried in Dallas County before Judge Jim Jordan. After a week and a half trial and two days of deliberation, the jury returned its verdict and found that Mr. Hoss had breached his fiduciary duty to Mr. Alardin. The jury awarded Mr. Alardin $3 million.
Michael Heygood, Partner of HORP&B, was the lead trial attorney for Tony Alardin, and was assisted at trial by Ryan Browne. Mr. Heygood commented, “At the end of the day, as is often the case, the jury was able to understand what was really going on.” According to Mr. Heygood, “Even though there was no written partnership agreement, the jury recognized the partnership between Mr. Hoss and Mr. Alardin and agreed that Mr. Hoss failed to treat Mr. Alardin fairly or justly."
Well done.
Another Fentanyl Recall (Actavis and Abrika)
Fentanyl patches were recalled for the second time in a week because of a problem that could cause patients or caregivers to overdose on the potent drug inside.
Sold in the United States by Actavis South Atlantic, the newly recalled patches have both this name and the company's former name, Abrika Pharmaceuticals, on their packaging. The old name is on the pouches that contain the patches and the new name is on the outer carton.
This recall includes 25-microgram-per-hour, 50-microgram-per-hour, 75 microgram-per-hour and 100 microgram-per-hour patches with expiration dates of May through August 2009.
What's wrong with the patches?
Several of the patches may have a defect that can cause them to leak. This would put both patients and caregivers at risk of coming into direct contact with the powerful drug inside the patch. This could result in difficulty breathing and a potentially fatal overdose.
Sold in the United States by Actavis South Atlantic, the newly recalled patches have both this name and the company's former name, Abrika Pharmaceuticals, on their packaging. The old name is on the pouches that contain the patches and the new name is on the outer carton.
This recall includes 25-microgram-per-hour, 50-microgram-per-hour, 75 microgram-per-hour and 100 microgram-per-hour patches with expiration dates of May through August 2009.
What's wrong with the patches?
Several of the patches may have a defect that can cause them to leak. This would put both patients and caregivers at risk of coming into direct contact with the powerful drug inside the patch. This could result in difficulty breathing and a potentially fatal overdose.
Monday, February 18, 2008
Could Drugmakers Use Journals to Promote Off Label Use?
From Bloomberg.com:
Drug and medical-device makers would be allowed to use medical journal articles to promote unapproved uses of their products under a U.S. proposal that drew immediate criticism on Capitol Hill.
The Food and Drug Administration issued draft guidelines today explaining the circumstances in which companies could distribute ``truthful and non-misleading'' articles to health- care providers.
Makers of drugs and devices aren't permitted to promote their products for conditions not approved by the FDA, although doctors are free to prescribe such ``off-label'' uses. Representative Henry Waxman, a California Democrat, said the FDA's proposal ``caters to the industry's desire'' to market products without enough testing or review.
Source here.
Drug and medical-device makers would be allowed to use medical journal articles to promote unapproved uses of their products under a U.S. proposal that drew immediate criticism on Capitol Hill.
The Food and Drug Administration issued draft guidelines today explaining the circumstances in which companies could distribute ``truthful and non-misleading'' articles to health- care providers.
Makers of drugs and devices aren't permitted to promote their products for conditions not approved by the FDA, although doctors are free to prescribe such ``off-label'' uses. Representative Henry Waxman, a California Democrat, said the FDA's proposal ``caters to the industry's desire'' to market products without enough testing or review.
Source here.
60 Minutes and Trasylol
From CBS:
"This is the story of a drug that was on the market for 14 years and may have contributed to the deaths of thousands of patients. Trasylol, made by Bayer, is given in the operating room to control bleeding. It was a big money maker.
As correspondent Scott Pelley reports, Bayer marketed Trasylol aggressively until it was used in about one third of all cardiac bypass operations in America.
But then, in 2006, a study showed widespread death associated with Trasylol, and as it turns out there was concern long before that.
How much did Bayer know? And why did it take Bayer and the U.S. Food and Drug Administration nearly two years to take the drug off the market after major studies revealed the danger? Two years - during which it's estimated Trasylol was contributing to the loss of one thousand lives a month. "
Video link here.
"This is the story of a drug that was on the market for 14 years and may have contributed to the deaths of thousands of patients. Trasylol, made by Bayer, is given in the operating room to control bleeding. It was a big money maker.
As correspondent Scott Pelley reports, Bayer marketed Trasylol aggressively until it was used in about one third of all cardiac bypass operations in America.
But then, in 2006, a study showed widespread death associated with Trasylol, and as it turns out there was concern long before that.
How much did Bayer know? And why did it take Bayer and the U.S. Food and Drug Administration nearly two years to take the drug off the market after major studies revealed the danger? Two years - during which it's estimated Trasylol was contributing to the loss of one thousand lives a month. "
Video link here.
Friday, February 15, 2008
Vytorin: Depression Added as a Side Effect
The FDA has approved a change in the product labels for cholesterol drugs Vytorin and Zetia, adding depression as a possible side effect of the drug.
In letters to the drugs' co-marketers, Schering-Plough Corp. (SGP) and Merck & Co. (MRK), the FDA said depression would be added to the section of the drugs' package insert concerning adverse reactions in post-marketing experience. The new language also will be included in a section of the patient package inserts listing possible side effects of the drugs.
The section of the product label being changed is for adverse reactions reported in post-marketing experience, "regardless of causality assessment." The FDA approved the label changes Feb. 7 and Feb. 8, its Web site indicates.
Vytorin is a combination of Zetia and simvastatin. Simvastatin is available generically and is marketed by Merck under the brand Zocor.
Link here.
In letters to the drugs' co-marketers, Schering-Plough Corp. (SGP) and Merck & Co. (MRK), the FDA said depression would be added to the section of the drugs' package insert concerning adverse reactions in post-marketing experience. The new language also will be included in a section of the patient package inserts listing possible side effects of the drugs.
The section of the product label being changed is for adverse reactions reported in post-marketing experience, "regardless of causality assessment." The FDA approved the label changes Feb. 7 and Feb. 8, its Web site indicates.
Vytorin is a combination of Zetia and simvastatin. Simvastatin is available generically and is marketed by Merck under the brand Zocor.
Link here.
Monday, February 11, 2008
FDA Warning for Botox
FDA Notifies Public of Adverse Reactions Linked to Botox Use
Ongoing safety review of Botox, Botox Cosmetic and Myobloc taking place
The U.S. Food and Drug Administration today notified the public that Botox and Botox Cosmetic (Botulinum toxin Type A) and Myobloc (Botulinum toxin Type B) have been linked in some cases to adverse reactions, including respiratory failure and death, following treatment of a variety of conditions using a wide range of doses.
In an early communication based on the FDA's ongoing safety review, the agency said the reactions may be related to overdosing. There is no evidence that these reactions are related to any defect in the products.
The adverse effects were found in FDA-approved and nonapproved usages. The most severe adverse effects were found in children treated for spasticity in their limbs associated with cerebral palsy. Treatment of spasticity is not an FDA-approved use of botulism toxins in children or adults.
The adverse reactions appear to be related to the spread of the toxin to areas distant from the site of injection, and mimic symptoms of botulism, which may include difficulty swallowing, weakness and breathing problems.
The FDA is not advising health care professionals to discontinue prescribing these products.
The agency is currently reviewing safety data from clinical studies submitted by the drugs' manufacturers, as well as post-marketing adverse event reports and medical literature. After completing a review of the data, the FDA will communicate to the public its conclusions, resulting recommendations, and any regulatory actions.
The notification is in keeping with the FDA's commitment to inform the public about its ongoing safety reviews of drugs. The early communication, which includes background information and advice for health care professionals, can be viewed at: http://www.fda.gov/cder/drug/early_comm/botulinium_toxins.htm
#
Ongoing safety review of Botox, Botox Cosmetic and Myobloc taking place
The U.S. Food and Drug Administration today notified the public that Botox and Botox Cosmetic (Botulinum toxin Type A) and Myobloc (Botulinum toxin Type B) have been linked in some cases to adverse reactions, including respiratory failure and death, following treatment of a variety of conditions using a wide range of doses.
In an early communication based on the FDA's ongoing safety review, the agency said the reactions may be related to overdosing. There is no evidence that these reactions are related to any defect in the products.
The adverse effects were found in FDA-approved and nonapproved usages. The most severe adverse effects were found in children treated for spasticity in their limbs associated with cerebral palsy. Treatment of spasticity is not an FDA-approved use of botulism toxins in children or adults.
The adverse reactions appear to be related to the spread of the toxin to areas distant from the site of injection, and mimic symptoms of botulism, which may include difficulty swallowing, weakness and breathing problems.
The FDA is not advising health care professionals to discontinue prescribing these products.
The agency is currently reviewing safety data from clinical studies submitted by the drugs' manufacturers, as well as post-marketing adverse event reports and medical literature. After completing a review of the data, the FDA will communicate to the public its conclusions, resulting recommendations, and any regulatory actions.
The notification is in keeping with the FDA's commitment to inform the public about its ongoing safety reviews of drugs. The early communication, which includes background information and advice for health care professionals, can be viewed at: http://www.fda.gov/cder/drug/early_comm/botulinium_toxins.htm
#
Friday, February 08, 2008
Text Messaging Privacy
Texting is omnipresent, and it's in the news quite a bit. Text messaging has become a huge part of people's lives, especially the under 30 set. How private it texting?
According to various sources, here is some news:
AT&T says it keep messages for up to 72 hours, Sprint PCS for two weeks, and Verizon says texts don't stay on the network for a long period.
Here are a few tips to keep texts private:
* Don't ever text personal information such as your PIN number, password, or banking information to anyone. Remember, once you send that information to another person, it gets stored in their cell phone and you don't want that.
* Put a password on your phone to keep others from accessing your text logs or email. This will also prevent thieves from stealing information stored in your phone.
* iPhone owners may want to change their SMS preview settings to make incoming text messaging more private. Apple iPhone Review has instructions on how to do this.
* Those concerned about privacy can send anonymous text messages with services like AnonTxt.com.
* Don't forget to erase all your personal data before selling, recycling or donating your old phone.
* And last but not least, remember that no matter how secure you think your carrier's SMS servers are, the ultimate security of private text messages depends on the recipient.
Source here.
According to various sources, here is some news:
AT&T says it keep messages for up to 72 hours, Sprint PCS for two weeks, and Verizon says texts don't stay on the network for a long period.
Here are a few tips to keep texts private:
* Don't ever text personal information such as your PIN number, password, or banking information to anyone. Remember, once you send that information to another person, it gets stored in their cell phone and you don't want that.
* Put a password on your phone to keep others from accessing your text logs or email. This will also prevent thieves from stealing information stored in your phone.
* iPhone owners may want to change their SMS preview settings to make incoming text messaging more private. Apple iPhone Review has instructions on how to do this.
* Those concerned about privacy can send anonymous text messages with services like AnonTxt.com.
* Don't forget to erase all your personal data before selling, recycling or donating your old phone.
* And last but not least, remember that no matter how secure you think your carrier's SMS servers are, the ultimate security of private text messages depends on the recipient.
Source here.
Did Lipitor Commercials use a Body Double for Dr. Jarvik?
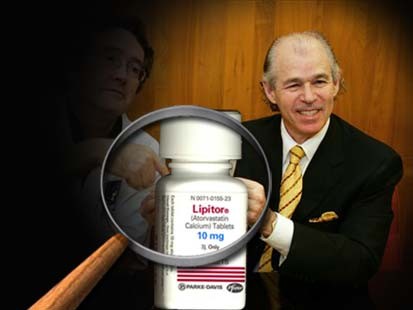
From various sources:
A congressional committee contends the creator of the first artificial heart could have have misled ad viewers by using a body double in advertisements for a popular cholesterol drug he touted in commercials. Yes you read that right, a body double.
Most folks have seen Dr. Robert Jarvik on the air in Lipitor commericals. Congressional investigators believe a body double was hired to make Jarvik look more athletic in television ads for the drug.
If that's not curious enough, Dr. Jarvik cannot legally prescribe medicine, because he is not licensed to practice medicine. Jarvik ended his training after medical school instead of completing a medical internship.
Sources include ABC News.
You can view the ad here:
Apparently the body double was used for the rowing segment, as opposed to the "standing by a labrador retriever" shot. Is the dog even his?
Thursday, February 07, 2008
Did we mean a $1B Zyprexa Settlement? Sorry, that was Confidential
The news that Eli Lilly was working out a deal with with federal and state prosecutors over improper marketing of Zyprexa was the result of an inadvertent leak from one of the lawyers on the matter.
A lawyer at Pepper Hamilton mistakenly sent an e-mail containing a confidential document to a reporter at The New York Times. Seems that the news reporter was named Alex Berenson. He has the same last name as another lawyer who was supposed to have received the e-mail, Bradford Berenson.
Some are calling it "A Nightmare on Email Street."
Thanks to several sites, including Fortune.
A lawyer at Pepper Hamilton mistakenly sent an e-mail containing a confidential document to a reporter at The New York Times. Seems that the news reporter was named Alex Berenson. He has the same last name as another lawyer who was supposed to have received the e-mail, Bradford Berenson.
Some are calling it "A Nightmare on Email Street."
Thanks to several sites, including Fortune.
Merck VP: Vytorin's News is a "Media Event"
From the WSJ Blog:
Ken Frazier from Merck spoke in New York City recently, and he addressed the Vytorin frenzy in the media in recent “We really feel that the Enhance data that has been put out into the public sphere has been mischaracterized,” he said.
Read more about Merck's view here.
Ken Frazier from Merck spoke in New York City recently, and he addressed the Vytorin frenzy in the media in recent “We really feel that the Enhance data that has been put out into the public sphere has been mischaracterized,” he said.
Read more about Merck's view here.
Monday, February 04, 2008
Medtronic SynchroMed EL Implantable Infusion Pump

Today's news brings news of another recall:
The FDA issued a Class I Recall of Medtronic Inc, SynchroMed EL Implantable Infusion Pump Models 8626-10, 8626L-10, 8626-18, 8626L-18, 8627-10, 8627L-10, 8627-18, and 8627L-18.
The device administers drugs to a specific site in the body to treat pain, spasticity (continuous muscle contraction), and cancer. The pump is implanted in the patient, either with or without a side catheter access port, catheters, and catheter accessories. The models were recalled because there is a potential pump motor stall issue that affects SynchroMed EL infusion pumps with motors manufactured before September 1999. If a pump motor stalls, drug delivery will stop suddenly and without warning. This stoppage will result in loss of therapy, return of the patient's symptoms, and/or symptoms of drug under infusion or withdrawal. Healthcare professionals and patients with questions should contact the manufacturer.
Go here for more.
FDA: Chantix Anti-smoking Drug Needs A Warning
Chantix looks to be linked to serious psychiatric behavior, including suicide. Chantix is a smoking cessation medicine.
The FDA reported that after an analysis of cases of depression, suicidal thoughts and other unusual behavior in patients on the medication, the evidence appears stronger of an association with Chantix.
For more go here.
The FDA reported that after an analysis of cases of depression, suicidal thoughts and other unusual behavior in patients on the medication, the evidence appears stronger of an association with Chantix.
For more go here.
Subscribe to:
Comments (Atom)